Diagnostic Accuracy of Endobronchial Optical Coherence Tomography for the Microscopic Diagnosis of Usual Interstitial Pneumonia
- PMID: 34375171
- PMCID: PMC8759308
- DOI: 10.1164/rccm.202104-0847OC
Diagnostic Accuracy of Endobronchial Optical Coherence Tomography for the Microscopic Diagnosis of Usual Interstitial Pneumonia
Abstract
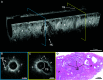
Rationale: Early, accurate diagnosis of interstitial lung disease (ILD) informs prognosis and therapy, especially in idiopathic pulmonary fibrosis (IPF). Current diagnostic methods are imperfect. High-resolution computed tomography has limited resolution, and surgical lung biopsy (SLB) carries risks of morbidity and mortality. Endobronchial optical coherence tomography (EB-OCT) is a low-risk, bronchoscope-compatible modality that images large lung volumes in vivo with microscopic resolution, including subpleural lung, and has the potential to improve the diagnostic accuracy of bronchoscopy for ILD diagnosis. Objectives: We performed a prospective diagnostic accuracy study of EB-OCT in patients with ILD with a low-confidence diagnosis undergoing SLB. The primary endpoints were EB-OCT sensitivity/specificity for diagnosis of the histopathologic pattern of usual interstitial pneumonia (UIP) and clinical IPF. The secondary endpoint was agreement between EB-OCT and SLB for diagnosis of the ILD fibrosis pattern. Methods: EB-OCT was performed immediately before SLB. The resulting EB-OCT images and histopathology were interpreted by blinded, independent pathologists. Clinical diagnosis was obtained from the treating pulmonologists after SLB, blinded to EB-OCT. Measurements and Main Results: We enrolled 31 patients, and 4 were excluded because of inconclusive histopathology or lack of EB-OCT data. Twenty-seven patients were included in the analysis (16 men, average age: 65.0 yr): 12 were diagnosed with UIP and 15 with non-UIP ILD. Average FVC and DlCO were 75.3% (SD, 18.5) and 53.5% (SD, 16.4), respectively. Sensitivity and specificity of EB-OCT was 100% (95% confidence interval, 75.8-100.0%) and 100% (79.6-100%), respectively, for both histopathologic UIP and clinical diagnosis of IPF. There was high agreement between EB-OCT and histopathology for diagnosis of ILD fibrosis pattern (weighted κ: 0.87 [0.72-1.0]). Conclusions: EB-OCT is a safe, accurate method for microscopic ILD diagnosis, as a complement to high-resolution computed tomography and an alternative to SLB.
Keywords: idiopathic pulmonary fibrosis; interstitial lung disease; in vivo microscopy; in vivo optical imaging; usual interstitial pneumonia.
Figures







Comment in
-
Endobronchial Optical Coherence Tomography for the Diagnosis of Fibrotic Interstitial Lung Disease: A Light at the End of the Tunnel?Am J Respir Crit Care Med. 2021 Nov 15;204(10):1122-1124. doi: 10.1164/rccm.202108-1899ED. Am J Respir Crit Care Med. 2021. PMID: 34473937 Free PMC article. No abstract available.
-
Endobronchial Optical Coherence Tomography: Shining New Light on Diagnosing Usual Interstitial Pneumonitis?Am J Respir Crit Care Med. 2022 Apr 15;205(8):967-968. doi: 10.1164/rccm.202111-2619LE. Am J Respir Crit Care Med. 2022. PMID: 35148494 Free PMC article. No abstract available.
References
-
- Daniil ZD, Gilchrist FC, Nicholson AG, Hansell DM, Harris J, Colby TV, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med . 1999;160:899–905. - PubMed
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. - PubMed
-
- Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society white paper. Lancet Respir Med . 2018;6:138–153. - PubMed
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083–2092. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2071–2082. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

