Identifying dominant-negative actions of a dopamine transporter variant in patients with parkinsonism and neuropsychiatric disease
- PMID: 34375312
- PMCID: PMC8492322
- DOI: 10.1172/jci.insight.151496
Identifying dominant-negative actions of a dopamine transporter variant in patients with parkinsonism and neuropsychiatric disease
Abstract
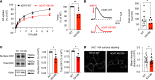
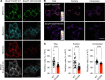
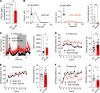
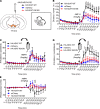
Dysfunctional dopaminergic neurotransmission is central to movement disorders and mental diseases. The dopamine transporter (DAT) regulates extracellular dopamine levels, but the genetic and mechanistic link between DAT function and dopamine-related pathologies is not clear. Particularly, the pathophysiological significance of monoallelic missense mutations in DAT is unknown. Here, we use clinical information, neuroimaging, and large-scale exome-sequencing data to uncover the occurrence and phenotypic spectrum of a DAT coding variant, DAT-K619N, which localizes to the critical C-terminal PSD-95/Discs-large/ZO-1 homology-binding motif of human DAT (hDAT). We identified the rare but recurrent hDAT-K619N variant in exome-sequenced samples of patients with neuropsychiatric diseases and a patient with early-onset neurodegenerative parkinsonism and comorbid neuropsychiatric disease. In cell cultures, hDAT-K619N displayed reduced uptake capacity, decreased surface expression, and accelerated turnover. Unilateral expression in mouse nigrostriatal neurons revealed differential effects of hDAT-K619N and hDAT-WT on dopamine-directed behaviors, and hDAT-K619N expression in Drosophila led to impairments in dopamine transmission with accompanying hyperlocomotion and age-dependent disturbances of the negative geotactic response. Moreover, cellular studies and viral expression of hDAT-K619N in mice demonstrated a dominant-negative effect of the hDAT-K619N mutant. Summarized, our results suggest that hDAT-K619N can effectuate dopamine dysfunction of pathological relevance in a dominant-negative manner.
Keywords: Cell Biology; Molecular genetics; Neuroscience; Parkinson disease; Psychiatric diseases.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

