Antiplatelet drugs block platelet activation by VITT patient serum
- PMID: 34375398
- PMCID: PMC8697531
- DOI: 10.1182/blood.2021012277
Antiplatelet drugs block platelet activation by VITT patient serum
Figures
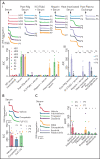
References
-
- Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. - PubMed
-
- MHRA - Medicines & Healthcare products Regulatory Agency . Coronavirus vaccine - weekly summary of Yellow Card reporting. 2021;1-14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

