The three Ts of virulence evolution during zoonotic emergence
- PMID: 34375554
- PMCID: PMC8354747
- DOI: 10.1098/rspb.2021.0900
The three Ts of virulence evolution during zoonotic emergence
Abstract
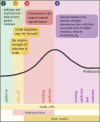
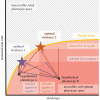
There is increasing interest in the role that evolution may play in current and future pandemics, but there is often also considerable confusion about the actual evolutionary predictions. This may be, in part, due to a historical separation of evolutionary and medical fields, but there is a large, somewhat nuanced body of evidence-supported theory on the evolution of infectious disease. In this review, we synthesize this evolutionary theory in order to provide a framework for clearer understanding of the key principles. Specifically, we discuss the selection acting on zoonotic pathogens' transmission rates and virulence at spillover and during emergence. We explain how the direction and strength of selection during epidemics of emerging zoonotic disease can be understood by a three Ts framework: trade-offs, transmission, and time scales. Virulence and transmission rate may trade-off, but transmission rate is likely to be favoured by selection early in emergence, particularly if maladapted zoonotic pathogens have 'no-cost' transmission rate improving mutations available to them. Additionally, the optimal virulence and transmission rates can shift with the time scale of the epidemic. Predicting pathogen evolution, therefore, depends on understanding both the trade-offs of transmission-improving mutations and the time scales of selection.
Keywords: emerging zoonotic disease; evolution; trade-offs; transmission; virulence.
Figures