Deployable ultrasound applicators for endoluminal delivery of volumetric hyperthermia
- PMID: 34376103
- PMCID: PMC8802621
- DOI: 10.1080/02656736.2021.1936216
Deployable ultrasound applicators for endoluminal delivery of volumetric hyperthermia
Abstract
Purpose: To investigate the design of an endoluminal deployable ultrasound applicator for delivering volumetric hyperthermia to deep tissue sites as a possible adjunct to radiation and chemotherapy.
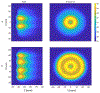
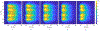
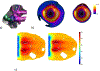
Method: This study considers an ultrasound applicator consisting of two tubular transducers situated at the end of a catheter assembly, encased within a distensible conical shaped balloon-based reflector that redirects acoustic energy distally into the tissue. The applicator assembly can be inserted endoluminally or laparoscopically in a compact form and expanded after delivery to the target site. Comprehensive acoustic and biothermal simulations and parametric studies were employed in generalized 3D and patient-specific pancreatic head and body tumor models to characterize the acoustic performance and evaluate heating capabilities of the applicator by investigating the device at a range of operating frequencies, tissue acoustic and thermal properties, transducer configurations, power modulation, applicator positioning, and by analyzing the resultant 40, 41, and 43 °C isothermal volumes and penetration depth of the heating volume. Intensity distributions and volumetric temperature contours were calculated to define moderate hyperthermia boundaries.
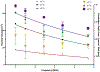
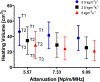
Results: Parametric studies demonstrated the frequency selection to control volume and depth of therapeutic heating from 62 to 22 cm3 and 4 to 2.6 cm as frequency ranges from 1 MHz to 4.7 MHz, respectively. Width of the heating profile tracks closely with the aperture. Water cooling within the reflector balloon was effective in controlling temperature to 37 °C maximum within the luminal wall. Patient-specific studies indicated that applicators with extended OD in the range of 3.6-6.2 cm with 0.5-1 cm long and 1 cm OD transducers can heat volumes of 1.1-7 cm3, 3-26 cm3, and 3.3-37.4 cm3 of pancreatic body and head tumors above 43, 41, and 40 °C, respectively.
Conclusion: In silico studies demonstrated the feasibility of combining endoluminal ultrasound with an integrated expandable balloon reflector for delivering volumetric hyperthermia in regions adjacent to body lumens and cavities.
Keywords: Ultrasound hyperthermia; endoluminal ultrasound applicator; pancreatic cancer; targeted drug delivery; thermal therapy.
Figures
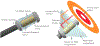


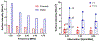



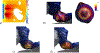
Similar articles
-
An endoluminal cylindrical sectored-ring ultrasound phased-array applicator for minimally-invasive therapeutic ultrasound.Med Phys. 2023 Jan;50(1):1-19. doi: 10.1002/mp.16113. Epub 2022 Dec 7. Med Phys. 2023. PMID: 36413363 Free PMC article.
-
Integration of deployable fluid lenses and reflectors with endoluminal therapeutic ultrasound applicators: Preliminary investigations of enhanced penetration depth and focal gain.Med Phys. 2017 Oct;44(10):5339-5356. doi: 10.1002/mp.12458. Epub 2017 Aug 8. Med Phys. 2017. PMID: 28681404 Free PMC article.
-
Thermal therapy of pancreatic tumours using endoluminal ultrasound: Parametric and patient-specific modelling.Int J Hyperthermia. 2016;32(2):97-111. doi: 10.3109/02656736.2015.1119892. Epub 2016 Jan 21. Int J Hyperthermia. 2016. PMID: 27097663 Free PMC article.
-
Catheter-based ultrasound applicators for selective thermal ablation: progress towards MRI-guided applications in prostate.Int J Hyperthermia. 2004 Nov;20(7):739-56. doi: 10.1080/02656730410001721816. Int J Hyperthermia. 2004. PMID: 15675669 Review.
-
Conformal microwave array (CMA) applicators for hyperthermia of diffuse chest wall recurrence.Int J Hyperthermia. 2010;26(7):686-98. doi: 10.3109/02656736.2010.501511. Int J Hyperthermia. 2010. PMID: 20849262 Free PMC article. Review.
Cited by
-
An endoluminal cylindrical sectored-ring ultrasound phased-array applicator for minimally-invasive therapeutic ultrasound.Med Phys. 2023 Jan;50(1):1-19. doi: 10.1002/mp.16113. Epub 2022 Dec 7. Med Phys. 2023. PMID: 36413363 Free PMC article.
-
Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer.Bioengineering (Basel). 2024 Dec 28;12(1):14. doi: 10.3390/bioengineering12010014. Bioengineering (Basel). 2024. PMID: 39851288 Free PMC article.
References
-
- Datta NR, et al., Enhanced tumour regression in a patient of liposarcoma treated with radiotherapy and hyperthermia: Hint for dynamic immunomodulation by hyperthermia. International Journal of Hyperthermia, 2015. 31(5): p. 574–577. - PubMed
-
- Hurwitz MD, Hyperthermia and immunotherapy: clinical opportunities. International Journal of Hyperthermia, 2019. 36(sup1): p. 4–9. - PubMed
-
- Wust P, et al., Hyperthermia in combined treatment of cancer. The Lancet Oncology, 2002. 3(8): p. 487–497. - PubMed
-
- Van Der Zee J, Heating the patient: a promising approach? Annals of Oncology, 2002. 13(8): p. 1173–1184. - PubMed
-
- Shakil A, Osborn JL, and Song CW, Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Radiat Oncol Biol Phys, 1999. 43(4): p. 859–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
