Performance of antigen testing for diagnosis of COVID-19: a direct comparison of a lateral flow device to nucleic acid amplification based tests
- PMID: 34376187
- PMCID: PMC8354301
- DOI: 10.1186/s12879-021-06524-7
Performance of antigen testing for diagnosis of COVID-19: a direct comparison of a lateral flow device to nucleic acid amplification based tests
Abstract
Objectives: The gold standard for diagnosing an infection with SARS-CoV-2 is detection of viral RNA by nucleic acid amplification techniques. Test capacities, however, are limited. Therefore, numerous easy-to-use rapid antigen tests based on lateral flow technology have been developed. Manufacturer-reported performance data seem convincing, but real-world data are missing.
Methods: We retrospectively analysed all prospectively collected antigen tests results performed between 23.06.2020 and 26.11.2020, generated by non-laboratory personnel at the point-of-care from oro- or nasopharyngeal swab samples at the University Hospital Augsburg and compared them to concomitantly (within 24 h.) generated results from molecular tests.
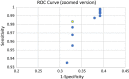
Results: For a total of 3630 antigen tests, 3110 NAAT results were available. Overall, sensitivity, specificity, NPV and PPV of antigen testing were 59.4%, 99.0%, 98.7% and 64.8%, respectively. Sensitivity and PPV were lower in asymptomatic patients (47.6% and 44.4%, respectively) and only slightly higher in patients with clinical symptoms (66.7% and 85.0%, respectively). Some samples with very low Ct-values (minimum Ct 13) were not detected by antigen testing. 31 false positive results occurred. ROC curve analysis showed that reducing the COI cut-off from 1, as suggested by the manufacturer, to 0.9 is optimal, albeit with an AUC of only 0.66.
Conclusion: In real life, performance of lateral-flow-based antigen tests are well below the manufacturer's specifications, irrespective of patient's symptoms. Their use for detection of individual patients infected with SARS-CoV2 should be discouraged. This does not preclude their usefulness in large-scale screening programs to reduce transmission events on a population-wide scale.
Keywords: (3–10): SARS-CoV-2; COVID-19 testing; Point-of-care testing; SARS-COV-2 antigen testing.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO CdC-tg. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases; Interim guidance 19 March 2020 2020. https://www.who.int/publications/i/item/10665-331501.
-
- WHO CdC-tg. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays 2020 https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous