Stability in eosinophil categorisation during subsequent severe exacerbations of COPD
- PMID: 34376399
- PMCID: PMC8354268
- DOI: 10.1136/bmjresp-2021-000960
Stability in eosinophil categorisation during subsequent severe exacerbations of COPD
Abstract
Background: The blood eosinophil count has been shown to be a promising biomarker for establishing personalised treatment strategies to reduce corticosteroid use, either inhaled or systemic, in chronic obstructive pulmonary disease (COPD). Eosinophil levels seem relatively stable over time in stable state, but little is known whether this is also true in subsequent severe acute exacerbations of COPD (AECOPD).
Aims and objectives: To determine the stability in eosinophil categorisation between two subsequent severe AECOPDs employing frequently used cut-off levels.
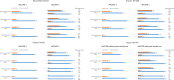
Methods: During two subsequent severe AECOPDs, blood eosinophil counts were determined at admission to the hospital in 237 patients in the Cohort of Mortality and Inflammation in COPD Study. The following four cut-off levels were analysed: absolute counts of eosinophils ≥0.2×10⁹/L (200 cells/µL) and ≥0.3×10⁹/L (300 cells/µL) and relative eosinophil percentage of ≥2% and ≥3% of total leucocyte count. Categorisations were considered stable if during the second AECOPD their blood eosinophil status led to the same classification: eosinophilic or not.
Results: Depending on the used cut-off, the overall stability in eosinophil categorisation varied between 70% and 85% during two subsequent AECOPDs. From patients who were eosinophilic at the first AECOPD, 34%-45% remained eosinophilic at the subsequent AECOPD, while 9%-21% of patients being non-eosinophilic at the first AECOPD became eosinophilic at the subsequent AECOPD.
Conclusions: The eosinophil variability leads to category changes in subsequent AECOPDs, which limits the eosinophil categorisation stability. Therefore, measurement of eosinophils at each new exacerbation seems warranted.
Keywords: COPD epidemiology; COPD exacerbations; eosinophil biology; pulmonary eosinophilia.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease . 2020 Global strategy for the prevention, diagnosis and management of COPD, 2020. Available: https://goldcopd.org/gold-reports/
-
- Sivapalan P, Lapperre TS, Janner J, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med 2019;7:699–709. 10.1016/S2213-2600(19)30176-6 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
