Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: an updated systematic review and meta-analysis
- PMID: 34376437
- PMCID: PMC8356177
- DOI: 10.1136/bmjopen-2020-043860
Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: an updated systematic review and meta-analysis
Abstract
Objective: Asthma often coexists with gastro-oesophageal reflux disease (GERD). The effect of proton pump inhibitors (PPIs) treatment on asthma concomitant with GERD was inconsistent. This study aimed to assess whether PPIs treatment improved morning peak expiratory flow (mPEF) in asthma patients with GERD.
Data sources: PubMed, MEDLINE, EMBASE, Web of Science, Cochrane Library and ClinicalTrials.gov; hand searching for reference lists; contacted with authors if necessary.
Study selection: All eligible trials were randomised clinical trials comparing PPIs with placebo in asthma patients accompanying with GERD.
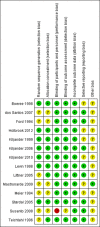
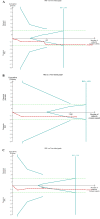
Results: Fourteen randomised clinical trials (2182 participants) were included. Overall, PPIs versus placebo did not affect mPEF in patients with asthma having GERD (weighted mean difference 8.68 L/min, 95% CI -2.02 to 19.37, p=0.11). Trial sequential analysis (TSA) further confirmed this finding (TSA adjusted 95% CI -1.03 to 22.25). Subgroups analyses based on the percentage of patients with symptomatic GERD≥95%, treatment duration >12 weeks also found no statistically significant benefit on mPEF. Similarly, analyses of secondary outcomes (evening PEF, forced expiratory volume in 1 s, asthma symptoms score, asthma quality of life score and episodes of asthma exacerbation) did not show significant difference between PPIs and placebo.
Conclusion: In this meta-analysis, PPIs therapy did not show a statistically significant improvement on mPEF in asthma patients having GERD, neither in subgroup with symptomatic GERD nor in subgroup with treatment duration >12 weeks. This analysis does not support a recommendation for PPIs therapy as empirical treatment in asthma patients with GERD.
Prospero registration number: CRD42020177330.
Keywords: asthma; oesophageal disease; therapeutics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
Effect of esomeprazole 40 mg once or twice daily on asthma: a randomized, placebo-controlled study.Am J Respir Crit Care Med. 2010 May 15;181(10):1042-8. doi: 10.1164/rccm.200910-1537OC. Epub 2010 Jan 28. Am J Respir Crit Care Med. 2010. PMID: 20110554 Clinical Trial.
-
Prevalence of gastro-esophageal reflux disease in patients with difficult to control asthma and effect of proton pump inhibitor therapy on asthma symptoms, reflux symptoms, pulmonary function and requirement for asthma medications.J Postgrad Med. 2014 Jul-Sep;60(3):282-6. doi: 10.4103/0022-3859.138754. J Postgrad Med. 2014. PMID: 25121368
-
Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease.Ann Pharmacother. 2010 Mar;44(3):572-6. doi: 10.1345/aph.1M519. Epub 2010 Feb 2. Ann Pharmacother. 2010. PMID: 20124466 Review.
-
[Effects of proton pump inhibitors in asthmatics with gastroesophageal reflux disease].Korean J Gastroenterol. 2011 Oct 25;58(4):178-83. doi: 10.4166/kjg.2011.58.4.178. Korean J Gastroenterol. 2011. PMID: 22042417 Korean.
-
Direct Comparison of the Efficacy and Safety of Vonoprazan Versus Proton-Pump Inhibitors for Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis.Dig Dis Sci. 2021 Jan;66(1):19-28. doi: 10.1007/s10620-020-06141-5. Epub 2020 Feb 24. Dig Dis Sci. 2021. PMID: 32095968
Cited by
-
Proton Pump Inhibitors in Allergy: Benefits and Risks.J Allergy Clin Immunol Pract. 2022 Dec;10(12):3117-3123. doi: 10.1016/j.jaip.2022.09.022. Epub 2022 Sep 23. J Allergy Clin Immunol Pract. 2022. PMID: 36162802 Free PMC article. Review.
-
Correlation of gastro-esophageal reflux disease with asthma control and quality of life: a cross-sectional study from a low-middle income country.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241297879. doi: 10.1177/17534666241297879. Ther Adv Respir Dis. 2024. PMID: 39512235 Free PMC article.
-
Causal relationship between gastroesophageal reflux disease and chronic obstructive respiratory disease: A bidirectional Mendelian randomization study.Heliyon. 2025 Jan 18;11(2):e42100. doi: 10.1016/j.heliyon.2025.e42100. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39906855 Free PMC article.
-
Pepsin and the Lung-Exploring the Relationship between Micro-Aspiration and Respiratory Manifestations of Gastroesophageal Reflux Disease.J Pers Med. 2022 Aug 7;12(8):1296. doi: 10.3390/jpm12081296. J Pers Med. 2022. PMID: 36013245 Free PMC article. Review.
-
The Saudi initiative for asthma - 2024 update: Guidelines for the diagnosis and management of asthma in adults and children.Ann Thorac Med. 2024 Jan-Mar;19(1):1-55. doi: 10.4103/atm.atm_248_23. Epub 2023 Dec 15. Ann Thorac Med. 2024. PMID: 38444991 Free PMC article.
References
-
- Global Initiative for Asthma . Global strategy for asthma management and prevention, 2019. Available: www.ginasthma.org
-
- Sandur V, Murugesh M, Banait V, et al. . Prevalence of gastro-esophageal reflux disease in patients with difficult to control asthma and effect of proton pump inhibitor therapy on asthma symptoms, reflux symptoms, pulmonary function and requirement for asthma medications. J Postgrad Med 2014;60:282–6. 10.4103/0022-3859.138754 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
