Patient-Reported Symptom Severity in a Nationwide Myasthenia Gravis Cohort: Cross-sectional Analysis of the Swedish GEMG Study
- PMID: 34376512
- PMCID: PMC8520390
- DOI: 10.1212/WNL.0000000000012604
Patient-Reported Symptom Severity in a Nationwide Myasthenia Gravis Cohort: Cross-sectional Analysis of the Swedish GEMG Study
Erratum in
-
Patient-Reported Symptom Severity in a Nationwide Myasthenia Gravis Cohort: Cross-sectional Analysis of the Swedish GEMG Study.Neurology. 2021 Dec 14;97(24):1141. doi: 10.1212/WNL.0000000000013021. Epub 2021 Oct 29. Neurology. 2021. PMID: 34716258 Free PMC article. No abstract available.
Abstract
Background and objectives: To describe myasthenia gravis activities of daily living (MG-ADL) in relation to clinical characteristics in a large Swedish nationwide cohort.
Methods: In a cross-sectional prevalence cohort study, the Genes and Environment in Myasthenia Gravis study, performed from November 2018 through August 2019, patients with myasthenia gravis (MG) were invited to submit an extensive 106-item life environment questionnaire, including the MG-ADL score. Patients were classified into early-onset MG (EOMG, <50 years), late-onset MG (LOMG, ≥50 years), or thymoma-associated MG (TAMG). Comparisons of disease-specific characteristics were made between subgroups, sexes, and different MG-ADL scores.
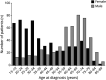
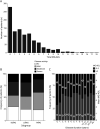
Results: A total of 1,077 patients were included, yielding a 74% response rate: 505 (47%) were classified as EOMG, 520 (48%) LOMG, and 45 (4%) TAMG. Mean age at inclusion was 64.3 years (SD 15.7) and mean disease duration was 14.6 years (SD 14.0). Complete MG-ADL scores (n = 1,035) ranged from 0p to 18p, where 26% reported a score of 0p. Higher MG-ADL scores were associated with female sex, obesity, and diagnostic delay (odds ratio [OR] 1.62, 1.72, and 1.69; p adj = 0.017, 0.013, and 0.008) and inversely correlated with high educational attainment (OR 0.59; p adj = 0.02), but not with age at inclusion, disease subtype, or disease duration. Almost half of the population (47%) reported MG-ADL ≥3p, corresponding to an unsatisfactory symptom state.
Discussion: In this nationwide study, comprising more than 40% of the prevalent MG population in Sweden, almost half of the patients reported current disease symptoms associated with an unsatisfactory symptom state, indicating the need for improved treatment options.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
People With Myasthenia Are Getting Better, but Are They Doing Well?Neurology. 2021 Oct 4;97(14):663-664. doi: 10.1212/WNL.0000000000012617. Neurology. 2021. PMID: 34376510 No abstract available.
Comment on
-
People With Myasthenia Are Getting Better, but Are They Doing Well?Neurology. 2021 Oct 4;97(14):663-664. doi: 10.1212/WNL.0000000000012617. Neurology. 2021. PMID: 34376510 No abstract available.
References
-
- Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. - PubMed
-
- Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis: autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259-268. - PubMed
-
- Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141-149. - PubMed
-
- Jones SM, Gwathmey KG, Burns TM. Quality of life measures for myasthenia gravis and evaluation of non-motor symptoms. Clin Exp Neuroimmunol. 2015;6(1):32-39.
-
- Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015;350:g7818. - PubMed
