Extracellular matrix mediates moruloid-blastuloid morphodynamics in malignant ovarian spheroids
- PMID: 34376568
- PMCID: PMC8358442
- DOI: 10.26508/lsa.202000942
Extracellular matrix mediates moruloid-blastuloid morphodynamics in malignant ovarian spheroids
Abstract
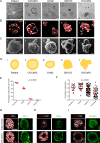
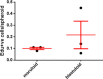
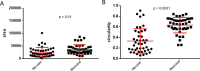
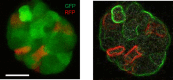
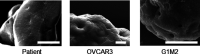
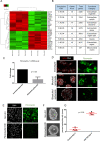
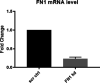
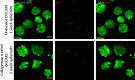
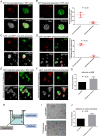
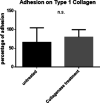
Ovarian cancer metastasizes into peritoneum through dissemination of transformed epithelia as multicellular spheroids. Harvested from the malignant ascites of patients, spheroids exhibit startling features of organization typical to homeostatic glandular tissues: lumen surrounded by smoothly contoured and adhered epithelia. Herein, we demonstrate that cells of specific ovarian cancer lines in suspension, aggregate into dysmorphic solid "moruloid" clusters that permit intercellular movement, cell penetration, and interspheroidal coalescence. Moruloid clusters subsequently mature into "blastuloid" spheroids with smooth contours, a temporally dynamic lumen and immotile cells. Blastuloid spheroids neither coalesce nor allow cell penetration. Ultrastructural examination reveals a basement membrane-like extracellular matrix coat on the surface of blastuloid, but not moruloid, spheroids. Quantitative proteomics reveals down-regulation in ECM protein Fibronectin-1 associated with the moruloid-blastuloid transition; immunocytochemistry also confirms the relocalization of basement membrane ECM proteins: collagen IV and laminin to the surface of blastuloid spheroids. Fibronectin depletion accelerates, and enzymatic basement membrane debridement impairs, lumen formation, respectively. The regulation by ECM dynamics of the morphogenesis of cancer spheroids potentially influences the progression of the disease.
© 2021 Langthasa et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
















References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
