VTA and Anterior Hippocampus Target Dissociable Neocortical Networks for Post-Novelty Enhancements
- PMID: 34376585
- PMCID: PMC8460145
- DOI: 10.1523/JNEUROSCI.0316-21.2021
VTA and Anterior Hippocampus Target Dissociable Neocortical Networks for Post-Novelty Enhancements
Abstract
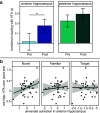
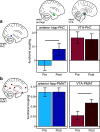
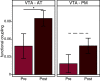
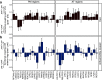
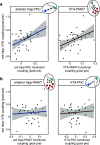
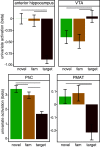
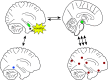
The detection of novelty indicates changes in the environment and the need to update existing representations. In response to novelty, interactions across the VTA-hippocampal circuit support experience-dependent plasticity in the hippocampus. While theories have broadly suggested plasticity-related changes are also instantiated in the cortex, research has also shown evidence for functional heterogeneity in cortical networks. It therefore remains unclear how the hippocampal-VTA circuit engages cortical networks, and whether novelty targets specific cortical regions or diffuse, large-scale cortical networks. To adjudicate the role of the VTA and hippocampus in cortical network plasticity, we used fMRI to compare resting-state functional coupling before and following exposure to novel scene images in human subjects of both sexes. Functional coupling between right anterior hippocampus and VTA was enhanced following novelty exposure. However, we also found evidence for a double dissociation, with anterior hippocampus and VTA showing distinct patterns of post-novelty functional coupling enhancements, targeting task-relevant regions versus large-scale networks, respectively. Further, significant correlations between these networks and the novelty-related plasticity in the anterior hippocampal-VTA functional network suggest that the central hippocampal-VTA network may facilitate the interactions with the cortex. These findings support an extended model of novelty-induced plasticity, in which novelty elicits plasticity-related changes in both local and global cortical networks.SIGNIFICANCE STATEMENT Novelty detection is critical for adaptive behavior, signaling the need to update existing representations. By engaging the bidirectional hippocampal-VTA circuit, novelty has been shown to induce plasticity-related changes in the hippocampus. However, it remains an open question how novelty targets such plasticity-related changes in cortical networks. We show that anterior hippocampus and VTA target cortical networks at different spatial scales, with respective enhancements in post-novelty functional coupling with a task-relevant cortical region and a large-scale memory network. The results presented here support an extended model of novelty-related plasticity, in which engaging the anterior hippocampal-VTA circuit through novelty exposure propagates cortical plasticity through hippocampal and VTA functional pathways at distinct scales, targeting specific or diffuse cortical networks.
Keywords: VTA; cortex; fMRI; functional coupling; hippocampus; novelty.
Copyright © 2021 the authors.
Figures

Similar articles
-
Sleep Spindles Promote the Restructuring of Memory Representations in Ventromedial Prefrontal Cortex through Enhanced Hippocampal-Cortical Functional Connectivity.J Neurosci. 2020 Feb 26;40(9):1909-1919. doi: 10.1523/JNEUROSCI.1946-19.2020. Epub 2020 Jan 20. J Neurosci. 2020. PMID: 31959699 Free PMC article.
-
Striatal and midbrain connectivity with the hippocampus selectively boosts memory for contextual novelty.Hippocampus. 2015 Nov;25(11):1262-73. doi: 10.1002/hipo.22434. Epub 2015 Apr 9. Hippocampus. 2015. PMID: 25708843 Free PMC article.
-
Amygdala and ventral tegmental area differentially interact with hippocampus and cortical medial temporal lobe during rest in humans.Hippocampus. 2020 Oct;30(10):1073-1080. doi: 10.1002/hipo.23216. Epub 2020 Jun 2. Hippocampus. 2020. PMID: 32485015 Free PMC article.
-
Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine.Hippocampus. 2001;11(5):551-68. doi: 10.1002/hipo.1071. Hippocampus. 2001. PMID: 11732708 Review.
-
The hippocampal-VTA loop: controlling the entry of information into long-term memory.Neuron. 2005 Jun 2;46(5):703-13. doi: 10.1016/j.neuron.2005.05.002. Neuron. 2005. PMID: 15924857 Review.
Cited by
-
Value restructures the organization of free recall.Cognition. 2023 Feb;231:105315. doi: 10.1016/j.cognition.2022.105315. Epub 2022 Nov 15. Cognition. 2023. PMID: 36399901 Free PMC article.
-
Sustained upregulation of widespread hippocampal-neocortical coupling following memory encoding.Cereb Cortex. 2023 Apr 4;33(8):4844-4858. doi: 10.1093/cercor/bhac384. Cereb Cortex. 2023. PMID: 36190442 Free PMC article.
-
Time-dependent memory transformation in hippocampus and neocortex is semantic in nature.Nat Commun. 2023 Sep 27;14(1):6037. doi: 10.1038/s41467-023-41648-1. Nat Commun. 2023. PMID: 37758725 Free PMC article.
-
Memory Boost for Recurring Emotional Events Is Driven by Initial Amygdala Response Promoting Stable Neocortical Patterns across Repetitions.J Neurosci. 2025 Apr 2;45(14):e2406232025. doi: 10.1523/JNEUROSCI.2406-23.2025. J Neurosci. 2025. PMID: 39947923 Free PMC article.
-
Stress disrupts insight-driven mnemonic reconfiguration in the medial temporal lobe.Neuroimage. 2023 Jan;265:119804. doi: 10.1016/j.neuroimage.2022.119804. Epub 2022 Dec 9. Neuroimage. 2023. PMID: 36503160 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
