In vivo multimodal retinal imaging of disease-related pigmentary changes in retinal pigment epithelium
- PMID: 34376700
- PMCID: PMC8355111
- DOI: 10.1038/s41598-021-95320-z
In vivo multimodal retinal imaging of disease-related pigmentary changes in retinal pigment epithelium
Abstract
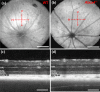
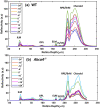
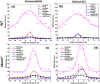
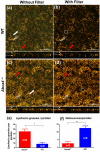
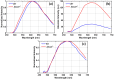
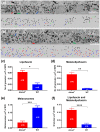
Melanosomes, lipofuscin, and melanolipofuscin are the three principal types of pigmented granules found in retinal pigment epithelium (RPE) cells. Changes in the density of melanosomes and lipofuscin in RPE cells are considered hallmarks of various retinal diseases, including Stargardt disease and age-related macular degeneration (AMD). Herein, we report the potential of an in vivo multimodal imaging technique based on directional back-scattering and short-wavelength fundus autofluorescence (SW-FAF) to study disease-related changes in the density of melanosomes and lipofuscin granules in RPE cells. Changes in the concentration of these granules in Abca4-/- mice (a model of Stargardt disease) relative to age-matched wild-type (WT) controls were investigated. Directional optical coherence tomography (dOCT) was used to assess melanosome density in vivo, whereas the autofluorescence (AF) images and emission spectra acquired with a spectrometer-integrated scanning laser ophthalmoscope (SLO) were used to characterize lipofuscin and melanolipofuscin granules in the same RPE region. Subcellular-resolution ex vivo imaging using confocal fluorescence microscopy and electron microscopy was performed on the same tissue region to visualize and quantify melanosomes, lipofuscin, and melanolipofuscin granules. Comparisons between in vivo and ex vivo results confirmed an increased concentration of lipofuscin granules and decreased concentration of melanosomes in the RPE of Abca4-/- mice, and provided an explanation for the differences in fluorescence and directionality of RPE scattering observed in vivo between the two mouse strains.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

