Spectral dynamic causal modelling in healthy women reveals brain connectivity changes along the menstrual cycle
- PMID: 34376799
- PMCID: PMC8355156
- DOI: 10.1038/s42003-021-02447-w
Spectral dynamic causal modelling in healthy women reveals brain connectivity changes along the menstrual cycle
Abstract
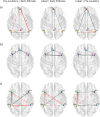
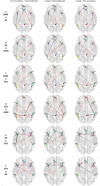
Longitudinal menstrual cycle studies allow to investigate the effects of ovarian hormones on brain organization. Here, we use spectral dynamic causal modelling (spDCM) in a triple network model to assess effective connectivity changes along the menstrual cycle within and between the default mode, salience and executive control networks (DMN, SN, and ECN). Sixty healthy young women were scanned three times along their menstrual cycle, during early follicular, pre-ovulatory and mid-luteal phase. Related to estradiol, right before ovulation the left insula recruits the ECN, while the right middle frontal gyrus decreases its connectivity to the precuneus and the DMN decouples into anterior/posterior parts. Related to progesterone during the mid-luteal phase, the insulae (SN) engage to each other, while decreasing their connectivity to parietal ECN, which in turn engages the posterior DMN. When including the most confident connections in a leave-one out cross-validation, we find an above-chance prediction of the left-out subjects' cycle phase. These findings corroborate the plasticity of the female brain in response to acute hormone fluctuations and may help to further understand the neuroendocrine interactions underlying cognitive changes along the menstrual cycle.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Tang, Y. et al. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb. Cortex10.1093/cercor/bhg121 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

