Gout-associated monosodium urate crystal-induced necrosis is independent of NLRP3 activity but can be suppressed by combined inhibitors for multiple signaling pathways
- PMID: 34376811
- PMCID: PMC9061757
- DOI: 10.1038/s41401-021-00749-7
Gout-associated monosodium urate crystal-induced necrosis is independent of NLRP3 activity but can be suppressed by combined inhibitors for multiple signaling pathways
Abstract
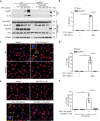
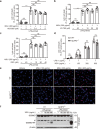
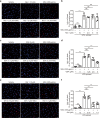
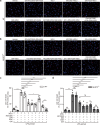
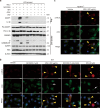
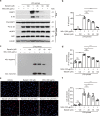
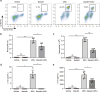
Monosodium urate (MSU) crystals, the etiological agent of gout, are formed in joints and periarticular tissues due to long-lasting hyperuricemia. Although MSU crystal-triggered NLRP3 inflammasome activation and interleukin 1β (IL-1β) release are known to have key roles in gouty arthritis, recent studies revealed that MSU crystal-induced necrosis also plays a critical role in this process. However, it remains unknown what forms of necrosis have been induced and whether combined cell death inhibitors can block such necrosis. Here, we showed that MSU crystal-induced necrosis in murine macrophages was not dependent on NLRP3 inflammasome activation, as neither genetic deletion nor pharmacological blockade of the NLRP3 pathway inhibited the necrosis. Although many cell death pathways (such as ferroptosis and pyroptosis) inhibitors or reactive oxygen species inhibitors did not have any suppressive effects, necroptosis pathway inhibitors GSK'872 (RIPK3 inhibitor), and GW806742X (MLKL inhibitor) dose-dependently inhibited MSU crystal-induced necrosis. Moreover, a triple combination of GSK'872, GW806742X, and IDN-6556 (pan-caspase inhibitor) displayed enhanced inhibition of the necrosis, which was further fortified by the addition of MCC950 (NLRP3 inhibitor), suggesting that multiple cell death pathways might have been triggered by MSU crystals. Baicalin, a previously identified inhibitor of NLRP3, inhibited MSU crystal-induced inflammasome activation and suppressed the necrosis in macrophages. Besides, baicalin gavage significantly ameliorated MSU crystal-induced peritonitis in mice. Altogether, our data indicate that MSU crystals induce NLRP3-independent necrosis, which can be inhibited by combined inhibitors for multiple signaling pathways, highlighting a new avenue for the treatment of gouty arthritis.
Keywords: baicalin; inflammasome; monosodium urate crystals; necroptosis; regulated necrosis.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling.J Cell Biochem. 2019 Apr;120(4):6718-6728. doi: 10.1002/jcb.27969. Epub 2018 Dec 28. J Cell Biochem. 2019. PMID: 30592318
-
Doliroside A attenuates monosodium urate crystals-induced inflammation by targeting NLRP3 inflammasome.Eur J Pharmacol. 2014 Oct 5;740:321-8. doi: 10.1016/j.ejphar.2014.07.023. Epub 2014 Jul 24. Eur J Pharmacol. 2014. PMID: 25064339
-
NLRP3 inflammasome inhibitor cucurbitacin B suppresses gout arthritis in mice.J Mol Endocrinol. 2021 Jun 21;67(2):27-40. doi: 10.1530/JME-20-0305. J Mol Endocrinol. 2021. PMID: 34047713
-
Role of NLRP3 in the pathogenesis and treatment of gout arthritis.Front Immunol. 2023 Mar 27;14:1137822. doi: 10.3389/fimmu.2023.1137822. eCollection 2023. Front Immunol. 2023. PMID: 37051231 Free PMC article.
-
Beneficial Properties of Phytochemicals on NLRP3 Inflammasome-Mediated Gout and Complication.J Agric Food Chem. 2018 Jan 31;66(4):765-772. doi: 10.1021/acs.jafc.7b05113. Epub 2018 Jan 17. J Agric Food Chem. 2018. PMID: 29293001 Review.
Cited by
-
Differences in macrophage pyroptosis and polarization induced by nano-/micro-calcium oxalate crystals.J Nanobiotechnology. 2025 Jul 10;23(1):499. doi: 10.1186/s12951-025-03549-x. J Nanobiotechnology. 2025. PMID: 40640899 Free PMC article.
-
Necroptosis in CNS diseases: Focus on astrocytes.Front Aging Neurosci. 2023 Jan 27;14:1016053. doi: 10.3389/fnagi.2022.1016053. eCollection 2022. Front Aging Neurosci. 2023. PMID: 36778591 Free PMC article. Review.
-
Leucocyte Abnormalities in Synovial Fluid of Degenerative and Inflammatory Arthropathies.Int J Mol Sci. 2023 Mar 13;24(6):5450. doi: 10.3390/ijms24065450. Int J Mol Sci. 2023. PMID: 36982526 Free PMC article.
-
Effects of Baicalin on Gout Based on Network Pharmacology, Molecular Docking, and in vitro Experiments.J Inflamm Res. 2025 Feb 4;18:1543-1556. doi: 10.2147/JIR.S480911. eCollection 2025. J Inflamm Res. 2025. PMID: 39925939 Free PMC article.
-
Necroptosis inhibitors: mechanisms of action and therapeutic potential.Apoptosis. 2024 Feb;29(1-2):22-44. doi: 10.1007/s10495-023-01905-6. Epub 2023 Nov 24. Apoptosis. 2024. PMID: 38001341 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

