Ascorbate is a multifunctional micronutrient whose synthesis is lacking in primates
- PMID: 34376908
- PMCID: PMC8325764
- DOI: 10.3164/jcbn.20-181
Ascorbate is a multifunctional micronutrient whose synthesis is lacking in primates
Abstract
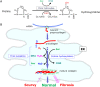
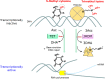
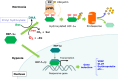
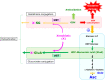
Ascorbate (vitamin C) is an essential micronutrient in primates, and exhibits multiple physiological functions. In addition to antioxidative action, ascorbate provides reducing power to α-ketoglutarate-dependent non-heme iron dioxygenases, such as prolyl hydroxylases. Demethylation of histones and DNA with the aid of ascorbate results in the reactivation of epigenetically silenced genes. Ascorbate and its oxidized form, dehydroascorbate, have attracted interest in terms of their roles in cancer therapy. The last step in the biosynthesis of ascorbate is catalyzed by l-gulono-γ-lactone oxidase whose gene Gulo is commonly mutated in all animals that do not synthesize ascorbate. One common explanation for this deficiency is based on the increased availability of ascorbate from foods. In fact, pathways for ascorbate synthesis and the detoxification of xenobiotics by glucuronate conjugation share the metabolic processes up to UDP-glucuronate, which prompts another hypothesis, namely, that ascorbate-incompetent animals might have developed stronger detoxification systems in return for their lack of ability to produce ascorbate, which would allow them to cope with their situation. Here, we overview recent advances in ascorbate research and propose that an enhanced glucuronate conjugation reaction may have applied positive selection pressure on ascorbate-incompetent animals, thus allowing them to dominate the animal kingdom.
Keywords: ascorbate; detoxification; glucuronate conjugation; vitamin C.
Copyright © 2021 JCBN.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




Similar articles
-
Ascorbate Is a Primary Antioxidant in Mammals.Molecules. 2022 Sep 21;27(19):6187. doi: 10.3390/molecules27196187. Molecules. 2022. PMID: 36234722 Free PMC article. Review.
-
L-gulono-γ-lactone oxidase expression and vitamin C synthesis in the brain and kidney of the African lungfish, Protopterus annectens.FASEB J. 2014 Aug;28(8):3506-17. doi: 10.1096/fj.14-249508. Epub 2014 Apr 25. FASEB J. 2014. PMID: 24769670
-
Vitamin C. Biosynthesis, recycling and degradation in mammals.FEBS J. 2007 Jan;274(1):1-22. doi: 10.1111/j.1742-4658.2006.05607.x. FEBS J. 2007. PMID: 17222174 Review.
-
L-ascorbic acid biosynthesis.Vitam Horm. 2001;61:241-66. doi: 10.1016/s0083-6729(01)61008-2. Vitam Horm. 2001. PMID: 11153268 Review.
-
Effect of vitamin C deficiency during postnatal development on adult behavior: functional phenotype of Gulo-/- knockout mice.Genes Brain Behav. 2012 Apr;11(3):269-77. doi: 10.1111/j.1601-183X.2011.00762.x. Epub 2012 Feb 2. Genes Brain Behav. 2012. PMID: 22296218 Free PMC article.
Cited by
-
Comparative analysis of phytochemicals and antioxidant activities in seeds and sprouts of different varieties of radish (Raphanus sativus L.): TOPSIS-entropy weight method.Front Plant Sci. 2025 Feb 7;16:1531570. doi: 10.3389/fpls.2025.1531570. eCollection 2025. Front Plant Sci. 2025. PMID: 39990715 Free PMC article.
-
Pleiotropic Actions of Aldehyde Reductase (AKR1A).Metabolites. 2021 May 26;11(6):343. doi: 10.3390/metabo11060343. Metabolites. 2021. PMID: 34073440 Free PMC article. Review.
-
A Clinically Relevant Dosage of Mitoxantrone Disrupts the Glutathione and Lipid Metabolic Pathways of the CD-1 Mice Brain: A Metabolomics Study.Int J Mol Sci. 2023 Aug 23;24(17):13126. doi: 10.3390/ijms241713126. Int J Mol Sci. 2023. PMID: 37685929 Free PMC article.
-
Ascorbate Is a Primary Antioxidant in Mammals.Molecules. 2022 Sep 21;27(19):6187. doi: 10.3390/molecules27196187. Molecules. 2022. PMID: 36234722 Free PMC article. Review.
-
Comparison of the effects of vitamin C and thiamine on refractory hypotension in patients with sepsis: A randomized controlled trial.Int J Crit Illn Inj Sci. 2022 Jul-Sep;12(3):138-145. doi: 10.4103/ijciis.ijciis_107_21. Epub 2022 Sep 20. Int J Crit Illn Inj Sci. 2022. PMID: 36506923 Free PMC article.

