Treatment Success Across Different Levels of Preoperative Disease Burden: Stratified Two-Year Outcomes from the Pivotal Trial of iStent inject ® Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract
- PMID: 34376967
- PMCID: PMC8349204
- DOI: 10.2147/OPTH.S316270
Treatment Success Across Different Levels of Preoperative Disease Burden: Stratified Two-Year Outcomes from the Pivotal Trial of iStent inject ® Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract
Abstract
Purpose: To examine effectiveness outcomes stratified by preoperative disease burden in the pivotal trial of iStent inject ® with cataract surgery (INJ) vs cataract surgery alone (CS).
Materials and methods: Prospective, 3:1 randomized, single-masked, concurrently-controlled, multicenter trial enrolling 505 subjects with cataract and mild-to-moderate primary open-angle glaucoma who underwent iStent inject implantation with phacoemulsification or phacoemulsification alone, and were followed for 2 years including annual medication washouts. Post hoc stratification was completed for baseline mean diurnal intraocular pressure (BL DIOP; Low-DIOP <25mmHg, Mid-DIOP ≥25 to <30 mmHg, High-DIOP ≥30mmHg) and preoperative medication burden (Low-Med 1 medication, Mid-Med 2 medications, High-Med ≥3 medications).
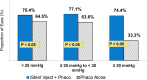
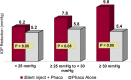
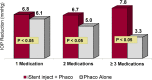
Results: The 24-month primary and secondary effectiveness endpoints were met, with significant treatment-over-control differences in percent of eyes achieving ≥20% unmedicated DIOP reduction and in unmedicated DIOP reduction, respectively. In subgroup analyses, the proportions of INJ eyes achieving the primary endpoint remained steady across all BL DIOP (75.4%, 77.1%, 74.4% in Low/Mid/High-DIOP strata, respectively) and preoperative medication levels (76.8%, 70.8%, 79.7% in Low/Mid/High-Med strata, respectively); meanwhile, the proportions of CS eyes diminished with higher BL DIOP (64.5%, 63.6%, 33.3%, respectively) and more medications (69.0%, 63.3%, 29.4%, respectively). Regarding secondary effectiveness, postoperative DIOP reduction increased with higher BL DIOP in INJ eyes (6.2mmHg, 7.8mmHg, 9.8mmHg, respectively) but plateaued in CS eyes (5.2mmHg, 5.8mmHg, 5.4mmHg, respectively). INJ eyes also had consistent DIOP reduction regardless of preoperative medication burden (6.8mmHg, 6.7mmHg, 7.8mmHg, respectively), while DIOP reduction diminished with more medications in CS eyes (6.1mmHg, 5.0mmHg, 3.3mmHg, respectively). Safety was favorable, comparable to phacoemulsification alone.
Conclusion: Significant IOP reductions occurred across all levels of BL DIOP and preoperative medication burden in iStent inject eyes. DIOP reductions increased with higher BL DIOP and remained stable across all levels of preoperative medication burden, suggesting the device's potential utility in more medically challenging cases.
Keywords: IOP; glaucoma; iStent inject; microinvasive glaucoma surgery/MIGS; severity; stratification; trabecular micro-bypass.
© 2021 Singh et al.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

