Efficacy and Safety of Teriflunomide in Multiple Sclerosis across Age Groups: Analysis from Pooled Pivotal and Real-world Studies
- PMID: 34377047
- PMCID: PMC8330455
- DOI: 10.1177/11795735211028781
Efficacy and Safety of Teriflunomide in Multiple Sclerosis across Age Groups: Analysis from Pooled Pivotal and Real-world Studies
Abstract
Background: Evidence suggests that efficacy and safety of disease-modifying treatments for multiple sclerosis may differ with age. We evaluate efficacy and safety of teriflunomide across age subgroups of patients from pooled clinical trials and real-world studies.
Methods: Post hoc analyses of patients who received teriflunomide 14 mg in the pooled phase II and III TEMSO, TOWER, TENERE, and TOPIC core and extension studies (n = 1978), and the real-world Teri-PRO (n = 928) and TAURUS-MS I (n = 1126) studies were conducted. Data were stratified by age at study entry: ⩽25, >25 to ⩽35, >35 to ⩽45, and >45 years. In Teri-PRO and TAURUS-MS I, an additional group, >55 years, was assessed.
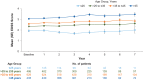
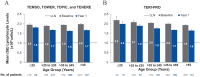
Results: In the pooled core studies, teriflunomide reduced annualized relapse rate (ARR) versus placebo across all ages. Unadjusted ARRs remained low across age groups in pooled extensions (0.18-0.30), Teri-PRO (0.10-0.35), and TAURUS-MS I (0.14-0.35). Baseline Expanded Disability Status Scale scores were higher with age, but stable through core and extension studies (mean increases over 7 years: ⩽25 years, +0.59; >25 to ⩽35 years, +0.46; >35 to ⩽45 years, +0.35; >45 years, +0.81). Across age groups, adverse event (AE) incidences were 78.4% to 90.7% in pooled core and extension studies and Teri-PRO, and 29.2% to 37.7% in TAURUS-MS I; serious AE incidences were ⩽21.3% in all studies. In pooled phase III and Teri-PRO studies, lymphocyte count decreases over 1 year after initiating teriflunomide, and proportions of patients developing lymphopenia, were small across age groups.
Conclusions: Teriflunomide efficacy was demonstrated regardless of age. Safety was generally consistent across age groups.
Keywords: Multiple sclerosis; central nervous system; demyelinating diseases.
© The Author(s) 2021.
Conflict of interest statement
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JO received consulting or speaking fees from Alexion, Biogen Idec, Celgene, EMD Serono, Genzyme, Novartis, and Roche, and received research support from Biogen Idec, EMD Serono, and Roche. SV has received grants, personal fees, and nonfinancial support from Biogen, Celgene, Genzyme, MedDay, Merck Serono, Novartis, Roche, Sanofi, and Teva. KT-W received honoraria for lectures, studies, and consultancy from Almirall, Bayer, Biogen, Genzyme, Ipsen, Merck Serono, Merz Pharma, Novartis, Roche, Sanofi, and Teva. JSI reports nothing to disclose. DR received consulting fees from Bayer, Biogen, Celgene, MedDay, Merck Serono, Novartis, Roche, Sanofi, and Teva, and received research support from Actelion, Biogen, Genzyme, GW Pharma, Merck Serono, Mitsubishi, Novartis, Teva, and TG Therapeutics. DPB is an employee of Sanofi with ownership interest. YP and EMP were employees of Sanofi at the time the analysis was done. PV received consulting and/or speaking fees, and research support from Biogen, Celgene, Merck Serono, Novartis, Roche, Sanofi, and Teva.
Figures

References
-
- Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129:595-605. - PubMed
LinkOut - more resources
Full Text Sources

