Cryptococcus extracellular vesicles properties and their use as vaccine platforms
- PMID: 34377375
- PMCID: PMC8329992
- DOI: 10.1002/jev2.12129
Cryptococcus extracellular vesicles properties and their use as vaccine platforms
Abstract
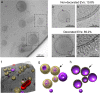
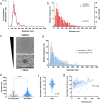
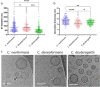
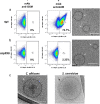
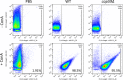
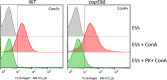
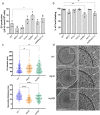
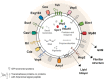
Whereas extracellular vesicle (EV) research has become commonplace in different biomedical fields, this field of research is still in its infancy in mycology. Here we provide a robust set of data regarding the structural and compositional aspects of EVs isolated from the fungal pathogenic species Cryptococcus neoformans, C. deneoformans and C. deuterogattii. Using cutting-edge methodological approaches including cryogenic electron microscopy and cryogenic electron tomography, proteomics, and flow cytometry, we revisited cryptococcal EV features and suggest a new EV structural model, in which the vesicular lipid bilayer is covered by mannoprotein-based fibrillar decoration, bearing the capsule polysaccharide as its outer layer. About 10% of the EV population is devoid of fibrillar decoration, adding another aspect to EV diversity. By analysing EV protein cargo from the three species, we characterized the typical Cryptococcus EV proteome. It contains several membrane-bound protein families, including some Tsh proteins bearing a SUR7/PalI motif. The presence of known protective antigens on the surface of Cryptococcus EVs, resembling the morphology of encapsulated virus structures, suggested their potential as a vaccine. Indeed, mice immunized with EVs obtained from an acapsular C. neoformans mutant strain rendered a strong antibody response in mice and significantly prolonged their survival upon C. neoformans infection.
Keywords: Cryo‐EM; cryptococcus; extracellular vesicles; fungal infections; mannoproteins; vaccine.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Figures

References
-
- Albuquerque, P. C. , Nakayasu, E S. , Rodrigues, M L. , Frases, S. , Casadevall, A. , Zancope‐Oliveira, R. M. , Almeida, I. C. , & Nosanchuk, J. D. (2008). Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans‐cell wall transfer of proteins and lipids in ascomycetes. Cellular Microbiology, 10, 1695–1710. - PMC - PubMed
-
- Armenteros, A. , Juan, J. , Tsirigos, K. D. , Sønderby, C. K. , Petersen, T. N. , Winther, O. , Brunak, S. , von Heijne, G. , & Nielsen, H. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37, 420–423. - PubMed
-
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12, 614–627. - PubMed
-
- Bachurski, D. , Schuldner, M. , Nguyen, P. ‐. H. , Malz, A. , Reiners, K. S. , Grenzi, P. C. , Babatz, F. , Schauss, A. C. , Hansen, H. P. , Hallek, M. , & Strandmann, E. P. (2019). Extracellular vesicle measurements with nanoparticle tracking analysis ‐ An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. Journal of Extracellular Vesicles, 8, 1596016–16. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

