Direct electrochemical hydrodefluorination of trifluoromethylketones enabled by non-protic conditions
- PMID: 34377412
- PMCID: PMC8336478
- DOI: 10.1039/d1sc01574e
Direct electrochemical hydrodefluorination of trifluoromethylketones enabled by non-protic conditions
Abstract
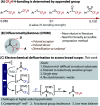
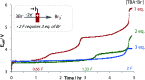
CF2H groups are unique due to the combination of their lipophilic and hydrogen bonding properties. The strength of H-bonding is determined by the group to which it is appended. Several functional groups have been explored in this context including O, S, SO and SO2 to tune the intermolecular interaction. Difluoromethyl ketones are under-studied in this context, without a broadly accessible method for their preparation. Herein, we describe the development of an electrochemical hydrodefluorination of readily accessible trifluoromethylketones. The single-step reaction at deeply reductive potentials is uniquely amenable to challenging electron-rich substrates and reductively sensitive functionality. Key to this success is the use of non-protic conditions enabled by an ammonium salt that serves as a reductively stable, masked proton source. Analysis of their H-bonding has revealed difluoromethyl ketones to be potentially highly useful dual H-bond donor/acceptor moieties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources

