The Role of the MCTS1 and DENR Proteins in Regulating the Mechanisms Associated with Malignant Cell Transformation
- PMID: 34377560
- PMCID: PMC8327141
- DOI: 10.32607/actanaturae.11181
The Role of the MCTS1 and DENR Proteins in Regulating the Mechanisms Associated with Malignant Cell Transformation
Abstract
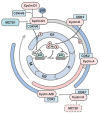
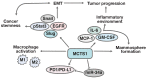
The mutations associated with malignant cell transformation are believed to disrupt the expression of a significant number of normal, non-mutant genes. The proteins encoded by these genes are involved in the regulation of many signaling pathways that are responsible for differentiation and proliferation, as well as sensitivity to apoptotic signals, growth factors, and cytokines. Abnormalities in the balance of signaling pathways can lead to the transformation of a normal cell, which results in tumor formation. Detection of the target genes and the proteins they encode and that are involved in the malignant transformation is one of the major evolutions in anti-cancer biomedicine. Currently, there is an accumulation of data that shed light on the role of the MCTS1 and DENR proteins in oncogenesis.
Keywords: MCTS1 and DENR proteins; apoptosis; cell cycle; malignant cell transformation; signaling pathways; translation initiation factors.
Copyright ® 2021 National Research University Higher School of Economics.
Figures



Similar articles
-
Identification of transcripts with short stuORFs as targets for DENR•MCTS1-dependent translation in human cells.Sci Rep. 2017 Jun 16;7(1):3722. doi: 10.1038/s41598-017-03949-6. Sci Rep. 2017. PMID: 28623304 Free PMC article.
-
DENR-MCTS1 heterodimerization and tRNA recruitment are required for translation reinitiation.PLoS Biol. 2018 Jun 11;16(6):e2005160. doi: 10.1371/journal.pbio.2005160. eCollection 2018 Jun. PLoS Biol. 2018. PMID: 29889857 Free PMC article.
-
DENR promotes translation reinitiation via ribosome recycling to drive expression of oncogenes including ATF4.Nat Commun. 2020 Sep 16;11(1):4676. doi: 10.1038/s41467-020-18452-2. Nat Commun. 2020. PMID: 32938922 Free PMC article.
-
ETS transcription factors and their emerging roles in human cancer.Eur J Cancer. 2005 Nov;41(16):2462-78. doi: 10.1016/j.ejca.2005.08.013. Epub 2005 Oct 6. Eur J Cancer. 2005. PMID: 16213704 Review.
-
The causes of cancer revisited: "mitochondrial malignancy" and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy.Mol Aspects Med. 2010 Apr;31(2):145-70. doi: 10.1016/j.mam.2010.02.008. Epub 2010 Mar 2. Mol Aspects Med. 2010. PMID: 20206201 Review.
Cited by
-
Postmortem muscle proteomics reveals breed specific responses to environmental enrichment and broiler meat quality.NPJ Sci Food. 2025 Jul 29;9(1):161. doi: 10.1038/s41538-025-00530-8. NPJ Sci Food. 2025. PMID: 40730582 Free PMC article.
-
Genome sequencing of 2000 canids by the Dog10K consortium advances the understanding of demography, genome function and architecture.Genome Biol. 2023 Aug 15;24(1):187. doi: 10.1186/s13059-023-03023-7. Genome Biol. 2023. PMID: 37582787 Free PMC article.
-
Long-term benefits of TUDCA supplement in ARSACS zebrafish model.Sci Rep. 2025 Jul 14;15(1):25429. doi: 10.1038/s41598-025-10850-0. Sci Rep. 2025. PMID: 40659819 Free PMC article.
References
-
- Prosniak M., Dierov J., Okami K., Tilton B., Jameson B., Sawaya B.E., Gartenhaus R.B.. Cancer Research. 1998;58(19):4233–4237. - PubMed
-
- Kasperaitis M.A.M., Voorma H.O., Thomas A.A.M.. FEBS Lett. 1995;365(1):47–50. - PubMed
-
- Reinert L.S., Shi B., Nandi S., Mazan-Mamczarz K., Vitolo M., Bachman K.E., He H., Gartenhaus R.B.. Cancer Research. 2006;66(18):8994–9001. - PubMed
LinkOut - more resources
Full Text Sources
