Hydrogen sulfide (H2S) signaling in plant development and stress responses
- PMID: 34377579
- PMCID: PMC7917380
- DOI: 10.1007/s42994-021-00035-4
Hydrogen sulfide (H2S) signaling in plant development and stress responses
Abstract
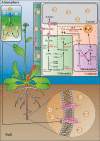
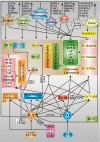
Abstract: Hydrogen sulfide (H2S) was initially recognized as a toxic gas and its biological functions in mammalian cells have been gradually discovered during the past decades. In the latest decade, numerous studies have revealed that H2S has versatile functions in plants as well. In this review, we summarize H2S-mediated sulfur metabolic pathways, as well as the progress in the recognition of its biological functions in plant growth and development, particularly its physiological functions in biotic and abiotic stress responses. Besides direct chemical reactions, nitric oxide (NO) and hydrogen peroxide (H2O2) have complex relationships with H2S in plant signaling, both of which mediate protein post-translational modification (PTM) to attack the cysteine residues. We also discuss recent progress in the research on the three types of PTMs and their biological functions in plants. Finally, we propose the relevant issues that need to be addressed in the future research.
Supplementary information: The online version contains supplementary material available at 10.1007/s42994-021-00035-4.
Keywords: Biotic and abiotic stresses; Growth and development; Hydrogen sulfide; Nitric oxide; Persulfidation; Reactive oxygen species; S-Nitrosylation; S-Sulfenylation; Sulfur metabolism.
© Agricultural Information Institute, Chinese Academy of Agricultural Sciences 2021.
Conflict of interest statement
Conflict of interestAll the authors state that there is no conflict of interest.
Figures


Similar articles
-
Interplay between hydrogen sulfide and other signaling molecules in the regulation of guard cell signaling and abiotic/biotic stress response.Plant Commun. 2021 Mar 15;2(3):100179. doi: 10.1016/j.xplc.2021.100179. eCollection 2021 May 10. Plant Commun. 2021. PMID: 34027393 Free PMC article. Review.
-
Hydrogen sulfide mechanism of action in plants; from interaction with regulatory molecules to persulfidation of proteins.Nitric Oxide. 2025 Jun;156:27-41. doi: 10.1016/j.niox.2025.02.001. Epub 2025 Feb 28. Nitric Oxide. 2025. PMID: 40024432 Review.
-
Expanding roles of cross-talk between hydrogen sulfide and nitric oxide under abiotic stress in plants.Plant Physiol Biochem. 2024 Sep;214:108852. doi: 10.1016/j.plaphy.2024.108852. Epub 2024 Jun 17. Plant Physiol Biochem. 2024. PMID: 38943878 Review.
-
Redox-based protein persulfidation in guard cell ABA signaling.Plant Signal Behav. 2020 May 3;15(5):1741987. doi: 10.1080/15592324.2020.1741987. Epub 2020 Mar 17. Plant Signal Behav. 2020. PMID: 32178559 Free PMC article.
-
The Interplay between Hydrogen Sulfide and Phytohormone Signaling Pathways under Challenging Environments.Int J Mol Sci. 2022 Apr 12;23(8):4272. doi: 10.3390/ijms23084272. Int J Mol Sci. 2022. PMID: 35457090 Free PMC article. Review.
Cited by
-
Improving salt tolerance of bean (Phaseolus vulgaris L.) with hydrogen sulfide.Photosynthetica. 2023 Feb 10;61(1):25-36. doi: 10.32615/ps.2023.005. eCollection 2023. Photosynthetica. 2023. PMID: 39650122 Free PMC article.
-
Antagonism of Rhizosphere Streptomyces yangpuensis CM253 against the Pathogenic Fungi Causing Corm Rot in Saffron (Crocus sativus L.).Pathogens. 2022 Oct 16;11(10):1195. doi: 10.3390/pathogens11101195. Pathogens. 2022. PMID: 36297252 Free PMC article.
-
Effect of hydrogen sulfide (H2S) on the growth and development of tobacco seedlings in absence of stress.BMC Plant Biol. 2024 Mar 2;24(1):162. doi: 10.1186/s12870-024-04819-w. BMC Plant Biol. 2024. PMID: 38429726 Free PMC article.
-
Biological Functions of Hydrogen Sulfide in Plants.Int J Mol Sci. 2022 Dec 1;23(23):15107. doi: 10.3390/ijms232315107. Int J Mol Sci. 2022. PMID: 36499443 Free PMC article. Review.
-
Exogenous hydrogen sulfide and methylglyoxal alleviate cadmium-induced oxidative stress in Salix matsudana Koidz by regulating glutathione metabolism.BMC Plant Biol. 2023 Feb 2;23(1):73. doi: 10.1186/s12870-023-04089-y. BMC Plant Biol. 2023. PMID: 36732696 Free PMC article.
References
-
- Aghdam MS, Mahmoudi R, Razavi F, Rabiei V, Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci Hort. 2018;238:264–271.
-
- Ahmad R, et al. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plantarum. 2020;168:289–300. - PubMed
-
- Al Ubeed HMS, Wills RBH, Bowyer MC, Golding JB. Inhibition of postharvest senescence of green leafy vegetables by exogenous D-cysteine and L-cysteine as precursors of hydrogen sulphide. J Hortic Sci Biotech. 2019;94:620–626.
-
- Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang GP. Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem. 2013;32:2234–2239. - PubMed
-
- Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou WJ. Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotox Environ Safe. 2014;110:197–207. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
