Rehabilitation and Return-to-Play Criteria After Fresh Osteochondral Allograft Transplantation: A Systematic Review
- PMID: 34377714
- PMCID: PMC8320585
- DOI: 10.1177/23259671211017135
Rehabilitation and Return-to-Play Criteria After Fresh Osteochondral Allograft Transplantation: A Systematic Review
Abstract
Background: Fresh osteochondral allograft (OCA) is a treatment option that allows for the transfer of size-matched allograft cartilage and subchondral bone into articular defects of the knee. Although long-term studies show good functional improvement with OCA, there continues to be wide variability and a lack of consensus in terms of postoperative rehabilitation protocols and return to sport.
Purpose: To systematically review the literature and evaluate the reported rehabilitation protocols after OCA of the knee, including weightbearing and range of motion (ROM) restrictions as well as return-to-play criteria.
Study design: Systematic review; Level of evidence, 4.
Methods: PubMed, EMBASE, Cumulative Index of Nursing Allied Health Literature, SPORTDiscus, and Cochrane databases were searched according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for studies on knee OCA. Studies were included if they reported return-to-play data or postsurgical rehabilitation protocols.
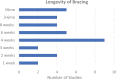
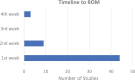
Results: A total of 62 studies met the inclusion criteria, with a total of 3451 knees in 3355 patients. Concomitant procedures were included in 30 of these studies (48.4%). The most commonly cited rehabilitation protocols included weightbearing restrictions and ROM guidelines in 100% and 90% of studies, respectively. ROM was most commonly initiated within the first postoperative week, with approximately half of studies utilizing continuous passive motion. Progression to weightbearing as tolerated was reported in 60 studies, most commonly at 6 weeks (range, immediately postoperatively to up to 1 year). Of the 62 studies, 37 (59.7%) included an expected timeline for either return to play or return to full activity, most commonly at 6 months (range, 4 months to 1 year). Overall, 13 studies (21.0%) included either objective or subjective criteria to determine return to activity within their rehabilitation protocol.
Conclusion: There is significant heterogeneity for postoperative rehabilitation guidelines and the return-to-play protocol after OCA of the knee in the literature, as nearly half of the included studies reported use of concomitant procedures. However, current protocols appear to be predominantly time-based without objective criteria or functional assessment. Therefore, the authors recommend the development of objective criteria for patient rehabilitation and return-to-play protocols after OCA of the knee.
Keywords: knee; osteochondral allograft; rehabilitation; return to play.
© The Author(s) 2021.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: B.T. has received research support from DePuy Synthes, GID, and Pacira; consulting fees and speaking fees from Mitek, DePuy Synthes, and Pacria; educational support from Vericel and Liberty Surgical; and hospitality payments from Stryker, Smith & Nephew, Medical Device Business Services, Flexion Therapeutics, and Dynasplint; and holds stock in Johnson & Johnson. S.H. is an employee and holds stock in Johnson & Johnson and has received educational support from Liberty Surgical and Arthrex and hospitality payments from Smith & Nephew, Aastrom Biosciences, DePuy Synthes, Horizon Pharma, Ferring Pharmaceuticals, Anika Therapeutics, and Vericel. K.B.F. has received consulting fees from DePuy Synthes, Vericel, and Medical Device Business Services; educational support from Liberty Surgical; honoraria, speaking fees, and nonconsulting fees from Vericel; and hospitality payments from Smith & Nephew, Ferring Pharmaceuticals, Arthrex, Cumberland Pharmaceuticals, and Flexion Therapeutics. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures


References
-
- Ackermann J, Merkely G, Shah N, Gomoll AH. Decreased graft thickness is associated with subchondral cyst formation after osteochondral allograft transplantation in the knee. Am J Sports Med. 2019;47(9):2123–2129. - PubMed
-
- Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 Years. Arthroscopy. 2016;32(10):2160–2168. - PubMed
-
- Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;391(suppl):S318–S327. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

