Emicizumab initiation and bleeding outcomes in people with hemophilia A with and without inhibitors: A single-center report
- PMID: 34377887
- PMCID: PMC8331949
- DOI: 10.1002/rth2.12571
Emicizumab initiation and bleeding outcomes in people with hemophilia A with and without inhibitors: A single-center report
Abstract
Background: Emicizumab, a bispecific antibody factor VIII mimetic, is approved for prophylaxis in hemophilia, and has different risks and side effects compared to factor VIII products.
Objective: To better understand the early impact of emicizumab on our patients at the University of Colorado Hemophilia and Thrombosis Center (UCHTC), we evaluated adverse reactions, factor prophylaxis overlap, and bleeding rates after starting emicizumab through a quality improvement project.
Patients/methods: A retrospective chart review and structured phone interview were conducted from June to September 2019 for all patients who had started emicizumab at the UCHTC. Data about emicizumab dosing, reactions, bleeding events, and bleeding treatment were collected in 68 children and adults (aged 0.55-79.8 years, on emicizumab a median 213 days; range, 51-1229 days) with hemophilia A (35.3% with past or current inhibitor).
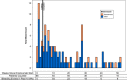
Results: Adverse reactions were primarily skin reactions, with no anaphylactic reactions or thrombosis. Bleeding events, defined as pain or swelling treated with factor or supportive measures, demonstrated wide variability, with 25 of 68 experiencing zero bleeds and 5 of 68 experiencing >8 bleeds per year. The most prevalent bleed type was traumatic musculoskeletal bleeding. Bleeding events occurred more often in the first 10 weeks after starting emicizumab, but no time period was without bleeding events. The majority of patients were prescribed every-week or every-2-week dosing, but some had alternative dosing frequency.
Conclusions: Real-world emicizumab use in our center was characterized by variations in prescribing practices and bleeding outcomes and lack of severe adverse reactions.
Keywords: bleeding; emicizumab; factor VIII; hemophilia; hemophilia A.
© 2021 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures

References
-
- Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130:2463‐2468. - PubMed
-
- Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811‐822. - PubMed
-
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. - PubMed
-
- MASAC . Recommendation on the use and management of emicizumab‐ KXWH (Hemlibra®) for hemophilia A with and without inhibitors. National Hemophilia Foundation; 2018.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

