MAF Amplification and Adjuvant Clodronate Outcomes in Early-Stage Breast Cancer in NSABP B-34 and Potential Impact on Clinical Practice
- PMID: 34377934
- PMCID: PMC8346694
- DOI: 10.1093/jncics/pkab054
MAF Amplification and Adjuvant Clodronate Outcomes in Early-Stage Breast Cancer in NSABP B-34 and Potential Impact on Clinical Practice
Abstract
Background: The Adjuvant Zoledronic Acid (ZA) study in early breast cancer (AZURE) showed correlation between a nonamplified MAF gene in the primary tumor and benefit from adjuvant ZA. Adverse ZA outcomes occurred in MAF-amplified patients. NSABP B-34 is a validation study.
Methods: A retrospective analysis of MAF gene status in NSABP B-34 was performed. Eligible patients were randomly assigned to standard adjuvant systemic treatment plus 3 years oral clodronate (1600 mg/daily) or placebo. Tumors were tested for MAF gene amplification and analyzed for their relationship to clodronate for disease-free survival (DFS) and overall survival (OS) in MAF nonamplified patients. All statistical tests were 2-sided .
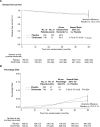
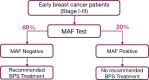
Results: MAF status was assessed in 2533 available primary tumor samples from 3311 patients. Of these, 37 withdrew consent; in 77 samples, no tumor was found; 536 assays did not meet quality standards, leaving 1883 (77.8%) evaluable for MAF assay by fluorescence in situ hybridization (947 from placebo and 936 from clodronate arms). At 5 years, in MAF nonamplified patients receiving clodronate, DFS improved by 30% (hazard ratio = 0.70, 95% confidence interval = 0.51 to 0.94; P = .02). OS improved at 5 years (hazard ratio = 0.59, 95% confidence interval = 0.37 to 0.93; P = .02) remaining statistically significant for clodronate throughout study follow-up. Conversely, adjuvant clodronate in women with MAF-amplified tumors was not associated with benefit but rather possible harm in some subgroups. Association between MAF status and menopausal status was not seen.
Conclusions: Nonamplified MAF showed statistically significant benefits (DFS and OS) with oral clodronate, supporting validation of the AZURE study.
© The Author(s) 2021. Published by Oxford University Press.
Figures

References
-
- Early Breast Cancer Trials Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet. 2015;386(10001):1353–1361. - PubMed
-
- Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: Consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016;27(3):379–390. - PubMed
-
- Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(18):2062–2081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
