Presence of ethanol-sensitive and ethanol-insensitive glycine receptors in the ventral tegmental area and prefrontal cortex in mice
- PMID: 34378188
- PMCID: PMC9293192
- DOI: 10.1111/bph.15649
Presence of ethanol-sensitive and ethanol-insensitive glycine receptors in the ventral tegmental area and prefrontal cortex in mice
Abstract
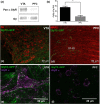
Background and purpose: Glycine receptors composed of α1 and β subunits are primarily found in the spinal cord and brainstem and are potentiated by ethanol (10-100 mM). However, much less is known about the presence, composition and ethanol sensitivity of these receptors in higher CNS regions. Here, we examined two regions of the brain reward system, the ventral tegmental area (VTA) and the prefrontal cortex (PFC), to determine their glycine receptor subunit composition and sensitivity to ethanol.
Experimental approach: We used Western blot, immunohistochemistry and electrophysiological techniques in three different models: wild-type C57BL/6, glycine receptor subunit α1 knock-in and glycine receptor subunit α2 knockout mice.
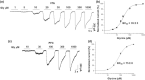
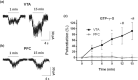
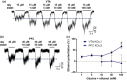
Key results: Similar levels of α and β receptor subunits were detected in both brain regions, and electrophysiological recordings demonstrated the presence of glycine-activated currents in both areas. Sensitivity of glycine receptors to glycine was lower in the PFC compared with VTA. Picrotoxin only partly blocked the glycine-activated current in the PFC and VTA, indicating that both regions express heteromeric αβ receptors. Glycine receptors in VTA neurons, but not in PFC neurons, were potentiated by ethanol.
Conclusion and implications: Glycine receptors in VTA neurons from WT and α2 KO mice were potentiated by ethanol, but not in neurons from the α1 KI mice, supporting the conclusion that α1 glycine receptors are predominantly expressed in the VTA. By contrast, glycine receptors in PFC neurons were not potentiated in any of the mouse models studied, suggesting the presence of α2/α3/α4, rather than α1 glycine receptor subunits.
Keywords: PFC; VTA; ethanol; glycine receptors; reward system; subunit composition.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Aguayo, L. G. , Castro, P. , Mariqueo, T. , Muñoz, B. , Xiong, W. , Zhang, L. , Lovinger, D. M. , & Homanics, G. E. (2014). Altered sedative effects of ethanol in mice with α1 glycine receptor subunits that are insensitive to Gβγ modulation. Neuropsychopharmacology, 39, 2538–2548. 10.1038/npp.2014.100 - DOI - PMC - PubMed
-
- Aguayo, L. G. , Tapia, J. C. , & Pancetti, F. C. (1996). Potentiation of the glycine‐activated Cl− current by ethanol in cultured mouse spinal neurons. The Journal of Pharmacology and Experimental Therapeutics, 279, 1116–1122. - PubMed
-
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 - DOI - PMC - PubMed
-
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. - PMC - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

