Histamine signaling and metabolism identify potential biomarkers and therapies for lymphangioleiomyomatosis
- PMID: 34378323
- PMCID: PMC8422079
- DOI: 10.15252/emmm.202113929
Histamine signaling and metabolism identify potential biomarkers and therapies for lymphangioleiomyomatosis
Abstract
Inhibition of mTOR is the standard of care for lymphangioleiomyomatosis (LAM). However, this therapy has variable tolerability and some patients show progressive decline of lung function despite treatment. LAM diagnosis and monitoring can also be challenging due to the heterogeneity of symptoms and insufficiency of non-invasive tests. Here, we propose monoamine-derived biomarkers that provide preclinical evidence for novel therapeutic approaches. The major histamine-derived metabolite methylimidazoleacetic acid (MIAA) is relatively more abundant in LAM plasma, and MIAA values are independent of VEGF-D. Higher levels of histamine are associated with poorer lung function and greater disease burden. Molecular and cellular analyses, and metabolic profiling confirmed active histamine signaling and metabolism. LAM tumorigenesis is reduced using approved drugs targeting monoamine oxidases A/B (clorgyline and rasagiline) or histamine H1 receptor (loratadine), and loratadine synergizes with rapamycin. Depletion of Maoa or Hrh1 expression, and administration of an L-histidine analog, or a low L-histidine diet, also reduce LAM tumorigenesis. These findings extend our knowledge of LAM biology and suggest possible ways of improving disease management.
Keywords: biomarker; histamine; lymphangioleiomyomatosis; mTOR; therapy.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
M.A.P. is recipient of an unrestricted research grant from Roche Pharma for the development of the ProCURE ICO research program.
Figures

Strategy for identifying metabolic plasma biomarkers and, subsequently, therapeutic opportunities for LAM based on the analysis of lung‐metastatic breast cancer gene expression data (Minn et al, 2005).
Significantly enriched metabolic pathways among the 30 enzymes identified in the previous analysis of TSC2 expression correlations.
Overabundance of MIAA in LAM plasma. Top panel shows each control and patient group (number (n) of samples are indicated); bottom panel shows aggregation of related pulmonary diseases. The asterisks indicate significant differences based on two‐sided Mann–Whitney tests (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; top panel, control‐LAM P = 0.048, LAM‐Langerhans P = 1 × 10−4, LAM‐Sjögren P = 1 × 10−4, LAM‐lupus P = 1 × 10−3, LAM‐emphysema P = 0.041; bottom panel, LAM‐Langerhans P = 7 × 10−6 and control‐other P = 0.031). Average values are indicated with lilac‐colored lines. Comparison of the three groups: Kruskal–Wallis test P = 6 × 10−4 (top panel) and P = 3 × 10−5 (bottom panel).
Lack of correlation between MIAA and VEGF‐D plasma measures (Spearman’s r s n.s., not significant).
Overabundance of MIAA in LAM patients not treated with rapamycin relative to rapamycin‐treated (number (n) of samples are indicated). The asterisk indicates significant difference based on two‐sided Mann–Whitney test (P = 0.043). Average values are indicated with lilac‐colored lines.
Receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC) and Akaike information criterion (AIC) values for the analyzed metabolites plus VEGF‐D, comparing LAM and healthy (left) or related pulmonary disease (right) plasma.
Plasma levels of three other metabolic products derived from monoamine metabolism and showing significant differences from controls and/or between LAM and related pulmonary diseases (individual samples indicated in panel C). The asterisks indicate significant differences based on two‐sided Mann–Whitney tests (*P < 0.05, **P < 0.01, and ****P < 0.0001; 4‐HPA, control‐LAM P = 0.024 and LAM‐other P = 0.089; HVA, control‐LAM P = 0.008 and LAM‐other P = 0.013; and VMA, control‐LAM P = 1 × 10−4 and LAM‐other P = 0.10). Average values are indicated with lilac‐colored lines. Detailed results by patient group are shown in Appendix Fig S1. Comparison of the three groups: Kruskal–Wallis test; 4‐HPA P = 0.095; HVA P = 0.013; and VMA P = 0.005.
Left panel, concise representation of the mitochondrial reactions mediated by MAOs and ALDHs from a monoamine (R‐NH2), to an aldehyde (R‐CHO), to the four identified metabolites (R‐COOH). The main monoamine origin of each metabolite is indicated in parentheses. Right panel, detail of the histamine metabolism pathway, with enzymes or enzymatic activities marked in red: ALDH, diamine oxidase (DAO), histidine decarboxylase (HDC), histamine N‐methyltransferase (HMT), imidazole acetic acid‐phosphoribosyl transferase (IPRT), and MAO‐B.

Overabundance of MIAA in LAM plasma (UK cohort not treated with rapamycin) relative to healthy women. The number (n) of individuals in each group is indicated; one of 20 healthy controls did not pass the quality controls for LC‐MS/MS. Asterisk indicates significance using two‐sided Mann–Whitney test (P = 0.018). Average values are indicated with lilac‐colored lines.
Overabundance of MIAA in plasma of LAM patients with AMLs. Asterisk indicates significance using two‐sided Mann–Whitney test (P = 0.042). The number (n) of individuals in each group is indicated. Average values are indicated with lilac‐colored lines.
Negative correlation (r s and P are indicated) between histamine levels in LAM plasma and patient FEV1 (% predicted), at the time of blood sample collection.
Similar negative correlation (rs and P are indicated) between histamine and DLCO.
Overabundance of MIAA in plasma of LAM patients with higher disease burden. Asterisk indicates significance using two‐sided Mann–Whitney test (P = 0.015). The number (n) of individuals in each group is indicated. Average values are indicated with lilac‐colored lines.
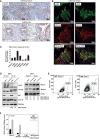
Representative images of immunohistochemical positivity of MAO‐A/B in LAM lung lesions (top panels, two patients) and lung tissue from healthy (non‐LAM) individuals (bottom panels). In total, seven LAM patients and three healthy controls were analyzed. Brown‐stained cells, counter‐stained with hematoxylin, are considered positive. Scale bars are shown.
Representative images of immunofluorescence detection of MAO‐A/B and αSMA in LAM lung lesions, nuclei stained with DAPI (merged).
Graph showing gene expression differences (log10‐fold changes) of defined genes (X‐axis) in Tsc2‐deficient relative to wild‐type MEFs grown in DMEM 10% FBS. The asterisks indicate significant differences with two‐sided t‐test (**P < 0.01, ***P < 0.001, and ****P < 0.0001; Aldh1a3 P = 0.006, Aldh2 P = 2 × 10−5, Aldh3a1 P = 0.004; Aldh3b1 P = 0.006; Aldh3b2, P = 0.17, Maoa P = 7 × 10−5, and Maob P = 7 × 10−4; replicates/condition n = 3, assays n = 4). The bars indicate mean ± SD. Dotted horizontal lines indicate 2‐fold (top) and 0.5‐fold (bottom).
Western blot results (independent experiments n = 4) from Tsc2‐deficient and wild‐type MEFs exposed to DMSO or treated with 20 nM everolimus for 16 h in DMEM 10% FBS. Loading controls are shown. The inferred protein expression level is indicated by the ratio between the corresponding signal and loading control, standardized to the basal setting (noted as 1 (reference (ref))).
Flow cytometry results (independent experiments n = 3) showing cell percentages for ALDEFLUOR‐positive MEFs grown in DMEM 10% FBS.
Quantified MAO basal activity (Y‐axis) in MEFs as depicted in the inset (grown in DMEM 10% FBS; replicates/condition n = 3 and independent experiments n = 2). The inhibitors (depicted on the X‐axis) were added to cell extracts prior to activity assay to assess the contribution of each MAO isoform. The significant difference corresponds to two‐way ANOVA test (P = 8 × 10−4). CPM: counts per minute. The bars indicate mean ± standard error of the mean (SEM).

No differences of Vdac1/VDAC1 expression between MEF cell lines (replicates/condition n = 3 and independent experiments n = 2). The graph shows fold change expression (mean ± SEM) in Tsc2‐deficient relative to wild‐type MEFs.
Flow cytometry results (independent experiments n = 3) showing a higher percentage of MitoSOX red‐positive cells in Tsc2‐deficient MEFs.
Flow cytometry results (independent experiments n = 3) showing higher intensity (X‐axis, single channel intensity FL3 670 nm) of MitoTracker red‐positive cells in Tsc2‐deficient MEFs.
Higher basal cell respiration (as measured by oxygen consumption, Y‐axis) in Tsc2‐deficient MEFs. The asterisks indicate significant difference with two‐sided t‐test (P = 5 × 10−4; replicates/condition n = 5, and independent experiments n = 3).
Higher hydroxide peroxide levels in Tsc2‐deficient MEFs across different numbers of seeded cells (X‐axis). Significance corresponds to two‐way ANOVA test (P = 1 × 10−4; replicates/condition n = 2, independent experiments n = 2). The bars indicate mean ± SD.
Left panel, Western blot results showing overexpression of catalase (CAT) in Tsc2‐deficient MEFs, and unaffected by exposure to everolimus (independent experiments n = 2). The CAT expression level is indicated by the ratio of the corresponding signal relative to loading control and basal setting (noted as 1(ref)). Right panel, Cat overexpression (log10‐fold change) in Tsc2‐deficient MEFs. The asterisks indicate significant difference with two‐sided t‐test (P = 1 × 10−2; replicates/condition n = 3, and independent experiments n = 2). The bars indicate mean ± SD. Dotted horizontal lines indicate 2‐fold (top) and 0.5‐fold (bottom).
Representative images of immunohistochemical detection of acrolein in LAM lung lesions. Seven LAM patients were analyzed, and assay controls are shown in Appendix Fig S2.

Overabundance of histamine in Tsc2 wild‐type relative to Tsc2‐deficient MEFs, both growth in DMEM 10% FBS. The asterisks indicate significant difference with two‐sided t‐test (P = 0.017); replicates/condition n = 5, and independent experiments (n = 2). Average values are indicated with lilac‐colored lines.
Isotope profiling of [13C6,15N3]‐labeled L‐histidine measured by LC‐MS/MS in Tsc2‐deficient and wild‐type MEFs grown in DMEM 10% FBS without unlabeled L‐histidine. Labeled‐measured metabolites are depicted in the X‐axis. The significant differences correspond to two‐sided t‐test (*P = 0.032 and **P = 0.008; replicates/condition n = 5). Average values are indicated with lilac‐colored lines.
Fold‐change variation of histamine and MIAA levels measured by LC‐MS/MS assays in rapamycin‐exposed (20 nM, 16 h) Tsc2‐deficient MEFs relative to DMSO‐exposed for the same period. The significant difference corresponds to one‐sided t‐test (P = 0.012; replicates/condition n = 4 and independent experiments n = 2). Average values are indicated with lilac‐colored lines.
Western blot results of HRH1, SLC22A3, and loading control from Tsc2‐deficient and wild‐type MEFs exposed to DMSO or treated with 20 nM everolimus for 16 h in DMEM 10% or 0.5% FBS (independent experiments n = 2). The expression levels are indicated by the ratio of the corresponding signal relative to loading control and basal setting (noted as 1(ref)).
Representative images of immunohistochemical detection of HRH1 in LAM lung lesions. A total of seven LAM patients were analyzed, and assay controls are shown in Appendix Fig S2.
Evaluation of cell viability inhibition by loratadine alone (concentrations shown on X‐axis, from 0 to 100 μM) or combined with everolimus (fixed to 20 nM) in Tsc2‐deficient and wild‐type MEF cultures grown in DMEM 10% FBS for 72 h. The synergistic combination index (CIx < 1) is shown (replicates/condition n = 3, independent experiments n = 3). Each data point represents the mean and SD.
Percentages of viability of Tsc2‐dificient MEFs exposed to increasing concentrations of histamine (X‐axis) in DMEM 0.5% FBS without L‐histidine for 72 h. The asterisk indicates significant difference with one‐sided t‐test (P = 0.040; replicates/condition n = 4–6, independent experiments n = 2). The bars indicate mean ± SD.
Percentages of viability of Tsc2‐dificient and wild‐type MEFs exposed to DMEM 10% or 0.5% FBS with or without L‐histidine for 72 h. The asterisks indicate significant differences with one‐sided t‐test (10% FBS, P = 0.017; 0.5% FBS, P = 5 × 10−4; replicates/condition n = 4–6, independent experiments n = 2). The bars indicate mean ± SD.
Western blot results of phospho‐Tyr783 PLCγ1 and loading control in Tsc2‐deficient MEFs grown in DMEM 0.5% FBS without L‐histidine and exposed to histamine (1 μM) for 10 min or 1 h. The signals of phospho‐Tyr783 PLCγ1 relative to loading control or non‐phospho PLCγ1 and to basal setting (noted as 1(ref)) are indicated.

LC‐MS/MS quantification of MIAA in plasma from C57BL/6 female mice with or without Tsc2‐deficient 105 K tumors grown. The asterisks indicate a significant difference with two‐sided t‐test (P = 8 × 10−3; replicates/condition n = 5). Average values are indicated with lilac‐colored lines.
Inhibition of Tsc2‐deficient 105 K tumor growth with different monotherapies, as indicated in the inset. Asterisks indicate significant reductions relative to vehicle (two‐way ANOVA; clorgyline P = 0.015, loratadine P = 0.018, and rasagiline P = 0.049). Each data point represents the mean and SEM.
Further reduction of Tsc2‐deficient 105 K tumor growth by rapamycin combined with clorgyline or loratadine, relative to rapamycin alone. Asterisks indicate significant reductions relative to rapamycin alone (two‐way ANOVA; clorgyline P = 0.035, loratadine P = 0.045). Each data point represents the mean and SEM.
Tsc2‐deficient 105 K tumor weight (g) changes at the end of the rapamycin and rapamycin‐combination assays. Asterisks indicate a significant reduction relative to control, as determined by a one‐sided t‐test (loratadine P = 0.009 and rapamycin P = 3 × 10−5). Average weight values are indicated with lilac‐colored lines.
Inhibition of Tsc2‐deficient 105 K tumor growth with Maoa expression depletion using shRNAs. Asterisks indicate significant reductions relative to control pLKO (two‐way ANOVA; P = 1 × 10−3). Each data point represents the mean and SEM.
Inhibition of Tsc2‐deficient 105K tumor growth with Hrh1 expression depletion. Asterisk indicates significant reduction relative to control pLKO (two‐way ANOVA; P = 0.035). Each data point represents the mean and SEM.
Inhibition of Tsc2‐deficient 105 K tumor growth with administration of α‐methyl‐DL‐histidine. Asterisk indicates significant reduction relative to vehicle (two‐way ANOVA; P = 0.028). Each data point represents the mean and SEM.
Inhibition of Tsc2‐deficient 105K tumor growth by the administration of a low L‐histidine concentration mouse diet (0.07% versus 0.49%). Asterisks indicate significant reduction relative to 0.49% L‐histidine diet (two‐way ANOVA; P = 0.001). Each data point represents the mean and SEM.
Increase in DLCO over a year in patients treated with rapamycin plus loratadine, relative to those treated with rapamycin alone. Significant difference determined by a two‐sided Mann–Whitney test (P = 0.045; number (n) of samples are indicated). Average values are indicated with lilac‐colored lines.
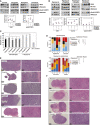
Top panels, Western blot results of HRH1 and phospho‐Ser616 DRP1 expression in vehicle control and single‐drug treated tumors. Bottom panels show quantifications; significant differences correspond to one‐sided t‐test (*P = 0.011 and **P = 2 × 10−3; tumors/group n = 4). Average values are indicated with lilac‐colored lines.
Western blot results showing increased HRH1 expression in combinations relative to rapamycin alone, reduced expression of S6 total in the rapamycin–loratadine combination, and raised phospho‐Ser616 DRP1 expression in this combination. Bottom panels show quantifications and significant differences with one‐sided t‐test (HRH1, clorgyline P = 0.011, loratadine P = 0.049, rasagiline P = 0.021; total S6, loratadine P = 4 × 10−4; phospho‐Ser616 DRP1, loratadine P = 0.037; tumors/group n = 4). Average values are indicated with lilac‐colored lines.
Graph showing the percentages of MitoSOX red‐positive Tsc2‐deficient 105 K cells treated for 24 h with DMSO or drugs in vitro with 10% FBS complete medium (except for the condition without L‐histidine). Clorgyline 1 μM, loratadine 100 nM, rasagiline 1 μM, and rapamycin 20 nM. Differences relative to rapamycin alone were determined by a two‐sided t‐test (DMSO P = 6 × 10−5, clorgyline P = 2 × 10−5, loratadine P = 6 × 10−4, rasagiline P = 4 × 10−5; combination with clorgyline P = 1 × 10−4, loratadine P = 3 × 10−5, and rasagiline P = 9 × 10−4; replicates/condition n = 5; assays n = 2). The bars indicate mean ± SD.
Results of blind histopathological evaluation of Tsc2‐deficient 105 K tumors treated with vehicle or drugs, in monotherapy or in combination with rapamycin. The histograms show the proportion of defined phenotypic scores: 1+, < 5% of the tumor; 2+, 5–50% of the tumor; and 3+, > 50% of the tumor. Cytological atypia was graded as mild (1+), moderate (2+), or severe (3+). Significant differences relative to rapamycin were determined using Fisher's exact test (epithelioid ***P = 9 × 10−4 and *P = 0.025; fibrosis, clorgyline and loratadine *P = 0.015, and rasagiline *P = 0.032; glandular, clorgyline *P = 0.030 and rasagiline *P = 0.045; and atypia, loratadine P = 0.010).
Representative images of hematoxylin–eosin‐stained Tsc2‐deficient 105K tumors treated with vehicle or monotherapies. Tumors treated with rapamycin tend to have a fascicular growth pattern with bundles of spindle cells and foci of fibrosis, whereas most of the tumors of the other treatment groups showed extensive epithelioid morphology. Scale bars are shown.
Representative images of hematoxylin–eosin‐stained Tsc2‐deficient 105K tumors treated with rapamycin alone or rapamycin combinations. With the addition of a second drug to rapamycin, tumors more frequently tended to show glandular differentiation and less atypia. Scale bars are shown.
References
-
- Bee J, Bhatt R, McCafferty I, Johnson SR (2015) A 4‐year prospective evaluation of protocols to improve clinical outcomes for patients with lymphangioleiomyomatosis in a national clinical centre. Thorax 70: 1202–1204 - PubMed
-
- Bee J, Fuller S, Miller S, Johnson SR (2018) Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: a prospective national cohort study. Thorax 73: 369–375 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

