Large organellar changes occur during mild heat shock in yeast
- PMID: 34378783
- PMCID: PMC8403982
- DOI: 10.1242/jcs.258325
Large organellar changes occur during mild heat shock in yeast
Abstract
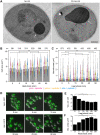
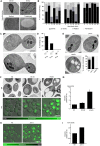
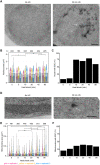
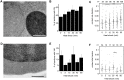
When the temperature is increased, the heat-shock response is activated to protect the cellular environment. The transcriptomics and proteomics of this process are intensively studied, while information about how the cell responds structurally to heat stress is mostly lacking. Here, Saccharomyces cerevisiae were subjected to a mild continuous heat shock (38°C) and intermittently cryo-immobilised for electron microscopy. Through measuring changes in all distinguishable organelle numbers, sizes and morphologies in over 2100 electron micrographs, a major restructuring of the internal architecture of the cell during the progressive heat shock was revealed. The cell grew larger but most organelles within it expanded even more, shrinking the volume of the cytoplasm. Organelles responded to heat shock at different times, both in terms of size and number, and adaptations of the morphology of some organelles (such as the vacuole) were observed. Multivesicular bodies grew by almost 70%, indicating a previously unknown involvement in the heat-shock response. A previously undescribed electron-translucent structure accumulated close to the plasma membrane. This all-encompassing approach provides a detailed chronological progression of organelle adaptation throughout the cellular heat-stress response.
Keywords: Budding yeast; Electron microscopy; Heat shock; Organelles; Ultrastructure.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Box, G. E. P. and Cox, D. R. (1964). An analysis of transformations revisited, rebutted. J. Am. Stat. Assoc. 26, 211-252.
-
- de Virgilio, C., Hottiger, T., Dominguez, J., Boller, T. and Wiemken, A. (1994). The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 219, 179-186. 10.1111/j.1432-1033.1994.tb19928.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

