Treatment patterns and outcomes for patients with newly diagnosed glioblastoma multiforme: a retrospective cohort study
- PMID: 34378977
- PMCID: PMC8461754
- DOI: 10.2217/cns-2021-0007
Treatment patterns and outcomes for patients with newly diagnosed glioblastoma multiforme: a retrospective cohort study
Abstract
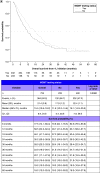
Aim: Investigate real-world outcomes and healthcare utilization of patients with glioblastoma multiforme (GBM) related to O6-methylguanine DNA methyltransferase (MGMT) promoter testing and methylation. Patients & methods: US Oncology Network data were analyzed for patients receiving first-line (1L) treatment for GBM. Results: Most patients received 1L radiation with temozolomide. Unadjusted median overall survival (OS) was higher in tested versus untested (median:18.1 vs 11.8 months) and in methylated versus unmethylated (median: 25.5 vs 12.4 months). Untested status, unmethylated MGMT and older age were associated with reduced OS and longer 1L treatment with increased OS. Similar findings were observed for progression-free survival. Utilization was similar between cohorts. Conclusion: In community oncology practices, MGMT methylation and testing were predictive of better survival in GBM.
Keywords: MGMT methylation; MGMT testing; glioblastoma; real-world evidence; survival.
Plain language summary
Lay abstract We studied the characteristics and survival of patients with newly diagnosed glioblastoma multiforme (GBM) in community-based oncology practices. These patients had received temozolomide and radiotherapy with surgery, which is the standard of care for GBM. We were interested in how patient survival was related to methylation of the O6-methylguanine DNA methyltransferase (MGMT) promoter. The study showed that patients with methylated versus unmethylated MGMT GBM survived longer. However, patients who were tested for methylation, whether MGMT was methylated or not, also survived longer. This may be because patients who get tested also get better care in general.
Conflict of interest statement
This work was supported by Bristol Myers Squibb. S Annavarapu employment: Ontada. A Gogate employment: Bristol Myers Squibb; stock and other ownership interests: Bristol Myers Squibb. T Pham employment: McKesson Specialty Health; stock and other ownership interests: McKesson. K Davies employment: McKesson Specialty Health. P Singh employment: Bristol Myers Squibb; stock and other ownership interests: Bristol Myers Squibb. N Robert employment: Ontada; leadership: Ontada; honoraria: Roche, Bristol Myers Squibb; consulting or advisory role: New Century Health, Bristol Myers Squibb, B Ingelheim; stock and other ownership interests: McKesson. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures




References
-
- Ostrom QT, Gittleman H, Liao Pet al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro. Oncol. 19(Suppl. 5), v1–v88 (2017). - PMC - PubMed
-
•• A systematic review and meta-analysis presenting trials of bevacizumab, tumor treating fields, and vaccines compared with standard of care for glioblastoma multiforme (GBM). So far, efficacy has not substantially improved with those therapies.
-
- Marenco-Hillembrand L, Wijesekera O, Suarez-Meade Pet al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J. Neurooncol. 147(2), 297–307 (2020). - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers Version 5.2020. (2021). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
-
- Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin. Cancer Res. 11(19 Pt 1), 6767–6771 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
