Tissue architecture delineates field cancerization in BRAFV600E-induced tumor development
- PMID: 34379110
- PMCID: PMC8380047
- DOI: 10.1242/dmm.048887
Tissue architecture delineates field cancerization in BRAFV600E-induced tumor development
Abstract
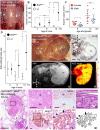
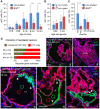
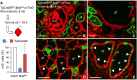
Cancer cells hijack developmental growth mechanisms but whether tissue morphogenesis and architecture modify tumorigenesis is unknown. Here, we characterized a new mouse model of sporadic thyroid carcinogenesis based on inducible expression of BRAF carrying a Val600 Glu (V600E) point mutation (BRAFV600E) from the thyroglobulin promoter (TgCreERT2). Spontaneous activation of this Braf-mutant allele due to leaky activity of the Cre recombinase revealed that intrinsic properties of thyroid follicles determined BRAF-mutant cell fate. Papillary thyroid carcinomas developed multicentrically within a normal microenvironment. Each tumor originated from a single follicle that provided a confined space for growth of a distinct tumor phenotype. Lineage tracing revealed oligoclonal tumor development in infancy and early selection of BRAFV600E kinase inhibitor-resistant clones. Somatic mutations were few, non-recurrent and limited to advanced tumors. Female mice developed larger tumors than males, reproducing the gender difference of human thyroid cancer. These data indicate that BRAFV600E-induced tumorigenesis is spatiotemporally regulated depending on the maturity and heterogeneity of follicles. Moreover, thyroid tissue organization seems to determine whether a BRAF-mutant lineage becomes a cancerized lineage. The TgCreERT2;BrafCA/+ sporadic thyroid cancer mouse model provides a new tool to evaluate drug therapy at different stages of tumor evolution.
Keywords: Braf mutation; Cancer; Development; Oligoclonal; Oncogenic activation; Thyroid.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




Similar articles
-
Tumor Cell Plasticity and Stromal Microenvironment Distinguish Papillary and Follicular Growth Patterns in a Mouse Model of BRAFV600E-Induced Thyroid Cancer.Cancer Res Commun. 2025 Mar 1;5(3):409-421. doi: 10.1158/2767-9764.CRC-24-0474. Cancer Res Commun. 2025. PMID: 39956582 Free PMC article.
-
Fine-Tuning Lipid Metabolism by Targeting Mitochondria-Associated Acetyl-CoA-Carboxylase 2 in BRAFV600E Papillary Thyroid Carcinoma.Thyroid. 2021 Sep;31(9):1335-1358. doi: 10.1089/thy.2020.0311. Epub 2021 Mar 3. Thyroid. 2021. PMID: 33107403 Free PMC article.
-
Mouse models of sporadic thyroid cancer derived from BRAFV600E alone or in combination with PTEN haploinsufficiency under physiologic TSH levels.PLoS One. 2018 Aug 7;13(8):e0201365. doi: 10.1371/journal.pone.0201365. eCollection 2018. PLoS One. 2018. PMID: 30086162 Free PMC article.
-
Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer.Endocr Relat Cancer. 2019 Jun;26(6):629-641. doi: 10.1530/ERC-17-0562. Endocr Relat Cancer. 2019. PMID: 30999281
-
The role of BRAF in the pathogenesis of thyroid carcinoma.Front Biosci (Landmark Ed). 2015 Jun 1;20(7):1068-78. doi: 10.2741/4359. Front Biosci (Landmark Ed). 2015. PMID: 25961545 Review.
Cited by
-
BRAFV600E Expression in Thyrocytes Causes Recruitment of Immunosuppressive STABILIN-1 Macrophages.Cancers (Basel). 2022 Sep 26;14(19):4687. doi: 10.3390/cancers14194687. Cancers (Basel). 2022. PMID: 36230610 Free PMC article.
-
Understanding and drugging RAS: 40 years to break the tip of the iceberg.Dis Model Mech. 2022 Feb 1;15(2):dmm049519. doi: 10.1242/dmm.049519. Epub 2022 Mar 4. Dis Model Mech. 2022. PMID: 35244677 Free PMC article.
-
First person - Elin Schoultz and Ellen Johansson.Dis Model Mech. 2022 Feb 1;15(2):dmm049155. doi: 10.1242/dmm.049155. Epub 2021 Aug 11. Dis Model Mech. 2022. PMID: 34379109 Free PMC article.
-
Tumor Cell Plasticity and Stromal Microenvironment Distinguish Papillary and Follicular Growth Patterns in a Mouse Model of BRAFV600E-Induced Thyroid Cancer.Cancer Res Commun. 2025 Mar 1;5(3):409-421. doi: 10.1158/2767-9764.CRC-24-0474. Cancer Res Commun. 2025. PMID: 39956582 Free PMC article.
-
Tissue specificity of oncogenic BRAF targeted to lung and thyroid through a shared lineage factor.iScience. 2023 Jun 8;26(7):107071. doi: 10.1016/j.isci.2023.107071. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534159 Free PMC article.
References
-
- Antico-Arciuch, V. G., Dima, M., Liao, X.-H., Refetoff, S. and Di Cristofano, A. (2010). Cross-talk between PI3K and estrogen in the mouse thyroid predisposes to the development of follicular carcinomas with a higher incidence in females. Oncogene 29, 5678-5686. 10.1038/onc.2010.308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

