Interventions for tophi in gout
- PMID: 34379791
- PMCID: PMC8406833
- DOI: 10.1002/14651858.CD010069.pub3
Interventions for tophi in gout
Abstract
Background: Tophi develop in untreated or uncontrolled gout. This is an update of a Cochrane Review first published in 2014. OBJECTIVES: To assess the benefits and harms of non-surgical and surgical treatments for the management of tophi in gout.
Search methods: We updated the search of Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase databases to 28 August 2020.
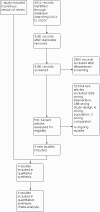
Selection criteria: We included all published randomised controlled trials (RCTs) or controlled clinical trials examining interventions for tophi in gout in adults.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
Main results: We included one trial in our original review. We added four more trials (1796 participants) in this update. One had three arms; pegloticase infusion every two weeks (biweekly), monthly pegloticase infusion (pegloticase infusion alternating with placebo infusion every two weeks) and placebo. Two studies looked at lesinurad 200 mg or 400 mg in combination with allopurinol. One trial studied lesinurad 200 mg or 400 mg in combination with febuxostat. One trial compared febuxostat 80 mg and 120 mg to allopurinol. Two trials were at unclear risk of performance and detection bias due to lack of information on blinding of participants and personnel. All other trials were at low risk of bias. Moderate-certainty evidence (downgraded for imprecision; one study; 79 participants) showed that biweekly pegloticase resolved tophi in 21/52 participants compared with 2/27 on placebo (risk ratio (RR) 5.45, 95% confidence interval (CI) 1.38 to 21.54; number needed to treat for a benefit (NNTB) 3, 95% CI 2 to 6). Similar proportions of participants receiving biweekly pegloticase (80/85) had an adverse event compared to placebo (41/43) (RR 0.99, 95% CI 0.91 to 1.07). However, more participants on biweekly pegloticase (15/85) withdrew due to an adverse event compared to placebo (1/43) (RR 7.59, 95% CI 1.04 to 55.55; number needed to treat for a harm (NNTH) 7, 95% CI 4 to 16). More participants on monthly pegloticase (11/52) showed complete resolution of tophi compared with placebo (2/27) (RR 2.86, 95% CI 0.68 to 11.97; NNTB 8, 95% CI 4 to 91). Similar numbers of participants on monthly pegloticase (84/84) had an adverse event compared to placebo (41/43) (RR 1.05, 95% CI 0.98 to 1.14). More participants on monthly pegloticase (16/84) withdrew due to adverse events compared to placebo (1/43) (RR 8.19, 95% CI 1.12 to 59.71; NNTH 6, 95% CI 4 to 14). Infusion reaction was the most common reason for withdrawal. Moderate-certainty evidence (2 studies; 103 participants; downgraded for imprecision) showed no clinically significant difference for complete resolution of target tophus in the lesinurad 200 mg plus allopurinol arm (11/53) compared to the placebo plus allopurinol arm (16/50) (RR 0.40, 95% CI 0.04 to 4.57), or in the lesinurad 400 mg plus allopurinol arm (12/48) compared to the placebo plus allopurinol arm (16/50) (RR 0.79, 95% CI 0.42 to 1.49). An extension study examined lesinurad 200 mg or 400 mg in combination with febuxostat, or placebo (low-certainty evidence, downgraded for indirectness and imprecision). Participants on lesinurad in the original study continued (CONT) on the same dose. Lesinurad 400 mg plus febuxostat may be beneficial for tophi resolution; 43/65 in the lesinurad 400 mg CONT arm compared to 38/64 in the lesinurad 200 mg CONT arm had tophi resolution (RR 1.11, 95% CI 0.85 to 1.46). Lesinurad 400 mg plus febuxostat may result in no difference in adverse events; 57/65 in the lesinurad 400 mg CONT arm had an adverse event compared to 50/64 in lesinurad 200 mg CONT arm (RR 1.12, 95% CI 0.96 to 1.32). Lesinurad 400 mg plus febuxostat may result in no difference in withdrawals due to adverse events; 10/65 participants in the lesinurad 400 mg CONT arm withdrew due to an adverse event compared to 10/64 participants in the lesinurad 200 mg CONT arm (RR 0.98, 95% CI 0.44 to 2.20). Lesinurad 400 mg plus febuxostat may result in no difference in mean serum uric acid (sUA), which was 3 mg/dl in the lesinurad 400 mg CONT group compared to 3.9 mg/dl in the lesinurad 200 mg CONT group (mean difference -0.90, 95% CI -1.51 to -0.29). Participants who were not on lesinurad in the original study were randomised (CROSS) to lesinurad 200 mg or 400 mg, both in combination with febuxostat. Low-certainty evidence downgraded for indirectness and imprecision showed that lesinurad 400 mg (CROSS) may result in tophi resolution (17/34) compared to lesinurad 200 mg (CROSS) (14/33) (RR 1.18, 95% CI 0.70 to 1.98). Lesinurad 400 mg in combination with febuxostat may result in no difference in adverse events (33/34 in the lesinurad 400 mg CROSS arm compared to 27/33 in the lesinurad 200 mg (CROSS); RR 1.19, 95% CI 1.00 to 1.41). Lesinurad 400 mg plus febuxostat may result in no difference in withdrawals due to adverse events, 5/34 in the lesinurad 400 mg CROSS arm withdrew compared to 2/33 in the lesinurad 200 mg CROSS arm (RR 2.43, 95% CI 0.51 to 11.64). Lesinurad 400 mg plus febuxostat results in no difference in sUA (4.2 mg/dl in lesinurad 400 mg CROSS) compared to lesinurad 200 mg (3.8 mg/dl in lesinurad 200 mg CROSS), mean difference 0.40 mg/dl, 95% CI -0.75 to 1.55.
Authors' conclusions: Moderate-certainty evidence showed that pegloticase is probably beneficial for resolution of tophi in gout. Although there was little difference in adverse events when compared to placebo, participants on pegloticase had more withdrawals due to adverse events. Lesinurad 400 mg plus febuxostat may be beneficial for tophi resolution compared with lesinurad 200 mg plus febuxostat; there was no difference in adverse events between these groups. We were unable to determine whether lesinurad plus febuxostat is more effective than placebo. Lesinurad (400 mg or 200 mg) plus allopurinol is probably not beneficial for tophi resolution, and there was no difference in adverse events between these groups. RCTs on interventions for managing tophi in gout are needed, and the lack of trial data is surprising given that allopurinol is a well-established treatment for gout.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MS none known
OV none known
JPP: none known
CB: none known
CE: none known
Figures

















Update of
-
Interventions for tophi in gout.Cochrane Database Syst Rev. 2014 Oct 20;(10):CD010069. doi: 10.1002/14651858.CD010069.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Aug 11;8:CD010069. doi: 10.1002/14651858.CD010069.pub3. PMID: 25330136 Updated.
Comment in
-
Are interventions for treatment of tophi in gout efficient? - A Cochrane Review summary with commentary.Int J Rheum Dis. 2023 Jul;26(7):1384-1387. doi: 10.1111/1756-185X.14648. Epub 2023 Mar 9. Int J Rheum Dis. 2023. PMID: 36891663 No abstract available.
References
References to studies included in this review
Bardin 2017 {published data only}
-
- Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Annals of the Rheumatic Diseases 2017;76(5):811-20. [DOI: 10.1136/annrheumdis-2016-209213] - DOI - PMC - PubMed
Becker 2005 {published data only}
-
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New England Journal of Medicine December 8, 2005;353(23):2450-61. - PubMed
Dalbeth 2019 {published data only}
Saag 2017 {published data only}
-
- Saag KG, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis & Rheumatology 2017;69(1):203-12. [DOI: 10.1002/art.39840] - DOI - PubMed
Sundy 2011 {published data only}
-
- Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment; two randomized controlled trials. Journal of the American Medical Association 2011;306:711-20. [ClinicalTrials.gov Identifier NCT00325195] - PubMed
References to studies excluded from this review
Doherty 2018 {published data only}
-
- Doherty M, Jenkins W, Richardson H, Sarmanova A, Abhishek A, Ashton D, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392(10156):1403-12. - PMC - PubMed
Fleischmann 2018 {published data only}
Haber 2018 {published data only}
-
- Haber SL, Fente G, Fenton SN, Walker EP, Weaver BM, Cano AJ, et al. Lesinurad: a novel agent for management of chronic gout. Annals of Pharmacotherapy 2018;52(7):690-6. - PubMed
Hao 2019 {published data only}
Hosoya 2018 {published data only}
Kankam 2018 {published data only}
Timilsina 2018 {published data only}
-
- Timilsina S, Brittan K, O'Dell JR, Brophy M, Davis-Karim A, Henrie AM, et al. Design and rationale for the Veterans Affairs "Cooperative study program 594 comparative effectiveness in gout: allopurinol vs. febuxostat" trial. Contemporary Clinical Trials 2018;68:102-8. - PubMed
White 2018 {published data only}
-
- White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al, CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. New England Journal of Medicine 2018;378(13):1200-10. - PubMed
References to ongoing studies
CLEAR I and II Extension {published data only}
-
- A long-term extension study of lesinurad in combination with allopurinol for subjects completing an efficacy and safety study of lesinurad and allopurinol. Ongoing study. February 2013. Contact author for more information.
DISSOLVE I {published data only}
-
- A study of SEL-212 in patients with gout refractory to conventional therapy (DISSOLVE I). Ongoing study. 18 August 2020. Contact author for more information.
DISSOLVE II {published data only}
-
- A study of SEL-212 in patients with gout refractory to conventional therapy II (DISSOLVE II). Ongoing study. October 2020. Contact author for more information.
MIRROR RCT {published data only}
-
- A randomized, double-Blind, placebo-controlled, multicenter, efficacy and safety study of methotrexate to increase response rates in patients with uncontrolled gout receiving KRYSTEXXA® (Pegloticase) (MIRROR Randomized Controlled Trial (RCT)). Ongoing study. 13 June 2019. Contact author for more information.
Additional references
Anderson 2010
Cates 2008
-
- Cates 2008. Visual Rx [Computer program]. Version 3; 2008. Available at: www.nntonline.net.
Covidence [Computer program]
-
- Covidence. Version accessed September 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
FitzGerald 2020
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed January 2021. Hamilton (ON): McMaster University (developed by EvidencePrime). Available at gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from training.cochrane.org/handbook/archive/v5.1/.
Khanna 2012
-
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al, American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research 2012;64(10):1431-46. - PMC - PubMed
Page 2020
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Schlesinger 2011
-
- Schlesinger N. Difficult-to-treat gouty arthritis: a disease warranting better management. Drugs 2011;71(11):1413-39. - PubMed
Schünemann 2020a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Schünemann 2020b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook..
Terkeltaub 2003
-
- Terkeltaub RA. Gout. New England Journal of Medicine 2003;349:1647-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

