Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis
- PMID: 34379914
- PMCID: PMC10662971
- DOI: 10.1056/NEJMoa2109908
Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis
Abstract
Background: Vaccine-induced immune thrombocytopenia and thrombosis (VITT) is a new syndrome associated with the ChAdOx1 nCoV-19 adenoviral vector vaccine against severe acute respiratory syndrome coronavirus 2. Data are lacking on the clinical features of and the prognostic criteria for this disorder.
Methods: We conducted a prospective cohort study involving patients with suspected VITT who presented to hospitals in the United Kingdom between March 22 and June 6, 2021. Data were collected with the use of an anonymized electronic form, and cases were identified as definite or probable VITT according to prespecified criteria. Baseline characteristics and clinicopathological features of the patients, risk factors, treatment, and markers of poor prognosis were determined.
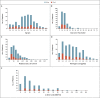
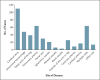
Results: Among 294 patients who were evaluated, we identified 170 definite and 50 probable cases of VITT. All the patients had received the first dose of ChAdOx1 nCoV-19 vaccine and presented 5 to 48 days (median, 14) after vaccination. The age range was 18 to 79 years (median, 48), with no sex preponderance and no identifiable medical risk factors. Overall mortality was 22%. The odds of death increased by a factor of 2.7 (95% confidence interval [CI], 1.4 to 5.2) among patients with cerebral venous sinus thrombosis, by a factor of 1.7 (95% CI, 1.3 to 2.3) for every 50% decrease in the baseline platelet count, by a factor of 1.2 (95% CI, 1.0 to 1.3) for every increase of 10,000 fibrinogen-equivalent units in the baseline d-dimer level, and by a factor of 1.7 (95% CI, 1.1 to 2.5) for every 50% decrease in the baseline fibrinogen level. Multivariate analysis identified the baseline platelet count and the presence of intracranial hemorrhage as being independently associated with death; the observed mortality was 73% among patients with platelet counts below 30,000 per cubic millimeter and intracranial hemorrhage.
Conclusions: The high mortality associated with VITT was highest among patients with a low platelet count and intracranial hemorrhage. Treatment remains uncertain, but identification of prognostic markers may help guide effective management. (Funded by the Oxford University Hospitals NHS Foundation Trust.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Clinical Features of VITT.N Engl J Med. 2022 Jan 6;386(1):10.1056/NEJMc2118473#sa1. doi: 10.1056/NEJMc2118473. Epub 2021 Dec 22. N Engl J Med. 2022. PMID: 34936756 No abstract available.
References
-
- Worldometer. Covid-19 coronavirus pandemic. July 8, 2021. (https://www.worldometers.info/coronavirus/).
-
- Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav 2021. May 10 (Epub ahead of print). - PubMed
-
- Gov.UK. First people to receive Oxford University/AstraZeneca COVID-19 vaccine today. Press release. January 7, 2021. (https://www.gov.uk/government/news/first-people-to-receive-oxford-univer...).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
