Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients
- PMID: 34379917
- PMCID: PMC8385563
- DOI: 10.1056/NEJMc2111462
Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients
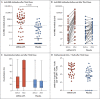
Figures
Comment in
- doi: 10.1056/NEJMe2112866
References
-
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205-1211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
