TDP-43 condensation properties specify its RNA-binding and regulatory repertoire
- PMID: 34380047
- PMCID: PMC8445024
- DOI: 10.1016/j.cell.2021.07.018
TDP-43 condensation properties specify its RNA-binding and regulatory repertoire
Abstract
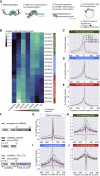
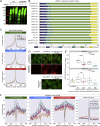
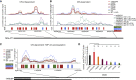
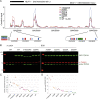
Mutations causing amyotrophic lateral sclerosis (ALS) often affect the condensation properties of RNA-binding proteins (RBPs). However, the role of RBP condensation in the specificity and function of protein-RNA complexes remains unclear. We created a series of TDP-43 C-terminal domain (CTD) variants that exhibited a gradient of low to high condensation propensity, as observed in vitro and by nuclear mobility and foci formation. Notably, a capacity for condensation was required for efficient TDP-43 assembly on subsets of RNA-binding regions, which contain unusually long clusters of motifs of characteristic types and density. These "binding-region condensates" are promoted by homomeric CTD-driven interactions and required for efficient regulation of a subset of bound transcripts, including autoregulation of TDP-43 mRNA. We establish that RBP condensation can occur in a binding-region-specific manner to selectively modulate transcriptome-wide RNA regulation, which has implications for remodeling RNA networks in the context of signaling, disease, and evolution.
Keywords: RNA granules; RNA-binding protein; TDP-43; alternative polyadenylation; amyotrophic lateral sclerosis; condensation; iCLIP; intrinsically disordered region; multivalency; phase separation.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.S. is a consultant for Dewpoint Therapeutics, Maze Therapeutics, and Vivid Sciences. B.P. is an employee of Dewpoint Therapeutics.
Figures



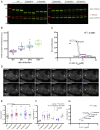
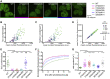
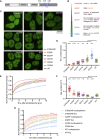
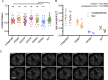



References
-
- Alberti S., Hyman A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021;22:196–213. - PubMed
-
- Astoricchio E., Alfano C., Rajendran L., Temussi P.A., Pastore A. The Wide World of Coacervates: From the Sea to Neurodegeneration. Trends Biochem. Sci. 2020;45:706–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FC001110 /MRC_/Medical Research Council/United Kingdom
- FC001110/WT_/Wellcome Trust/United Kingdom
- FC001110 /CRUK_/Cancer Research UK/United Kingdom
- T32 GM132039/GM/NIGMS NIH HHS/United States
- R01 NS116176/NS/NINDS NIH HHS/United States
- 215593/Z/19/Z /WT_/Wellcome Trust/United Kingdom
- 110292/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- 103760/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- 101149/Z/13/A/WT_/Wellcome Trust/United Kingdom
- MR/S006591/1/MRC_/Medical Research Council/United Kingdom
- FC001110 /WT_/Wellcome Trust/United Kingdom
- HALLEGGER/OCT15/959-799/MNDA_/Motor Neurone Disease Association/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

