Acute toxicity assessment for TiO2 photocatalyst (GST) made from wastewater using TiCl4 in rat
- PMID: 34380292
- PMCID: PMC8598407
- DOI: 10.5620/eaht.2021019
Acute toxicity assessment for TiO2 photocatalyst (GST) made from wastewater using TiCl4 in rat
Abstract
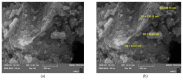
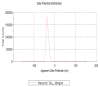
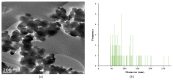
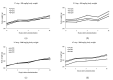
TiO2 was a photocatalyst that used to the most common product because of the high efficiency. TiO2 (P-25, commercial nanomaterial product) is the most typical photocatalyst product and TiO2 (GST) was a sludge recycling product. This study was reported to evaluate an acute toxicity of TiO2 (P-25 and GST) according to OECD test guideline 402 and 423 in Sprague-Dawley (SD) female rats via route of oral and dermal. There was investigated the lethal dose (LD50), and mortality, clinical signs, body weight changes and gross findings were continually monitored for 14 days following the single administration. After administration, TiO2 (P-25) was calculated that LD50 was considered to be a dose of over 2000 mg/kg body weight for both different route of exposure, and TiO2 (GST) was the same. Other items were no observed an adverse effect between P-25 and GST; no mortality and clinical signs, accidental body weight loss, no gross findings. On the basis of the above results, the toxicity of the GST was almost equal to that of the commercial product, P-25 and there was no toxicological evidence.
Keywords: GST; P-25; TiO2; dermal; nanomaterial; oral.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Moma J. Modified titanium dioxide for photocatalytic applications. Photocatalysts-Applications and Attributes. 2019:18. doi: 10.5772/intechopen.79374. - DOI
-
- Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B Environ. 2012;125:331–349. doi: 10.1016/j.apcatb.2012.05.036. - DOI
-
- Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev. 2000;1(1):1–21. doi: 10.1016/S1389-5567(00)00002-2. - DOI
-
- Jiang L, Wang Y, Feng C. Application of photocatalytic technology in environmental safety. Procedia Engineering. 2012;45:993–997. doi: 10.1016/j.proeng.2012.08.271. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

