Protein detection in blood with single-molecule imaging
- PMID: 34380620
- PMCID: PMC8357237
- DOI: 10.1126/sciadv.abg6522
Protein detection in blood with single-molecule imaging
Abstract
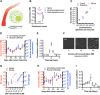
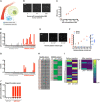
The ability to characterize individual biomarker protein molecules in patient blood samples could enable diagnosis of diseases at an earlier stage, when treatment is typically more effective. Single-molecule imaging offers a promising approach to accomplish this goal. However, thus far, single-molecule imaging methods have not been translated into the clinical setting. The detection limit of these methods has been confined to the picomolar (10-12 M) range, several orders of magnitude higher than the circulating concentrations of biomarker proteins present in many diseases. Here, we describe single-molecule augmented capture (SMAC), a single-molecule imaging technique to quantify and characterize individual protein molecules of interest down to the subfemtomolar (<10-15 M) range. We demonstrate SMAC in a variety of applications with human blood samples, including the analysis of disease-associated secreted proteins, membrane proteins, and rare intracellular proteins. SMAC opens the door to the application of single-molecule imaging in noninvasive disease profiling.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Barletta J. M., Edelman D. C., Constantine N. T., Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am. J. Clin. Pathol. 122, 20–27 (2004). - PubMed
-
- Srinivas P. R., Kramer B. S., Srivastava S., Trends in biomarker research for cancer detection. Lancet Oncol. 2, 698–704 (2001). - PubMed
-
- Rissin D. M., Kan C. W., Campbell T. G., Howes S. C., Fournier D. R., Song L., Piech T., Patel P. P., Chang L., Rivnak A. J., Ferrell E. P., Randall J. D., Provuncher G. K., Walt D. R., Duffy D. C., Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010). - PMC - PubMed
-
- Nam J.-M., Thaxton C. S., Mirkin C. A., Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301, 1884–1886 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

