GIP Receptor Agonism Attenuates GLP-1 Receptor Agonist-Induced Nausea and Emesis in Preclinical Models
- PMID: 34380697
- PMCID: PMC8564411
- DOI: 10.2337/db21-0459
GIP Receptor Agonism Attenuates GLP-1 Receptor Agonist-Induced Nausea and Emesis in Preclinical Models
Abstract
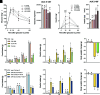
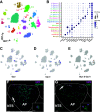
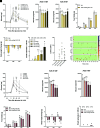
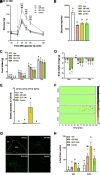
Glucagon-like peptide 1 receptor (GLP-1R) agonists decrease body weight and improve glycemic control in obesity and diabetes. Patient compliance and maximal efficacy of GLP-1 therapeutics are limited by adverse side effects, including nausea and emesis. In three different species (i.e., mice, rats, and musk shrews), we show that glucose-dependent insulinotropic polypeptide receptor (GIPR) signaling blocks emesis and attenuates illness behaviors elicited by GLP-1R activation, while maintaining reduced food intake, body weight loss, and improved glucose tolerance. The area postrema and nucleus tractus solitarius (AP/NTS) of the hindbrain are required for food intake and body weight suppression by GLP-1R ligands and processing of emetic stimuli. Using single-nuclei RNA sequencing, we identified the cellular phenotypes of AP/NTS cells expressing GIPR and GLP-1R on distinct populations of inhibitory and excitatory neurons, with the greatest expression of GIPR in γ-aminobutyric acid-ergic neurons. This work suggests that combinatorial pharmaceutical targeting of GLP-1R and GIPR will increase efficacy in treating obesity and diabetes by reducing nausea and vomiting.
© 2021 by the American Diabetes Association.
Figures
References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

