Hypothalamic Glutamate/GABA Cotransmission Modulates Hippocampal Circuits and Supports Long-Term Potentiation
- PMID: 34380766
- PMCID: PMC8482861
- DOI: 10.1523/JNEUROSCI.0410-21.2021
Hypothalamic Glutamate/GABA Cotransmission Modulates Hippocampal Circuits and Supports Long-Term Potentiation
Abstract
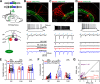
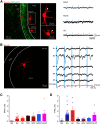
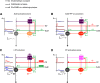
Subcortical input engages in cortico-hippocampal information processing. Neurons of the hypothalamic supramammillary nucleus (SuM) innervate the dentate gyrus (DG) by coreleasing two contrasting fast neurotransmitters, glutamate and GABA, and thereby support spatial navigation and contextual memory. However, the synaptic mechanisms by which SuM neurons regulate the DG activity and synaptic plasticity are not well understood. The DG comprises excitatory granule cells (GCs) as well as inhibitory interneurons (INs). Combining optogenetic, electrophysiological, and pharmacological approaches, we demonstrate that the SuM input differentially regulates the activities of different DG neurons in mice of either sex via distinct synaptic mechanisms. Although SuM activation results in synaptic excitation and inhibition in all postsynaptic cells, the ratio of these two components is variable and cell type-dependent. Specifically, dendrite-targeting INs receive predominantly synaptic excitation, whereas soma-targeting INs and GCs receive primarily synaptic inhibition. Although SuM excitation alone is insufficient to excite GCs, it enhances the GC spiking precision and reduces the latencies in response to excitatory drives. Furthermore, SuM excitation enhances the GC spiking in response to the cortical input, thereby promoting induction of long-term potentiation at cortical-GC synapses. Collectively, these findings provide physiological significance of the cotransmission of glutamate/GABA by SuM neurons in the DG network.SIGNIFICANCE STATEMENT The cortical-hippocampal pathways transfer mnemonic information during memory acquisition and retrieval, whereas subcortical input engages in modulation of communication between the cortex and hippocampus. The supramammillary nucleus (SuM) neurons of the hypothalamus innervate the dentate gyrus (DG) by coreleasing glutamate and GABA onto granule cells (GCs) and interneurons and support memories. However, how the SuM input regulates the activity of various DG cell types and thereby contributes to synaptic plasticity remains unexplored. Combining optogenetic and electrophysiological approaches, we demonstrate that the SuM input differentially regulates DG cell dynamics and consequently enhances GC excitability as well as synaptic plasticity at cortical input-GC synapses. Our findings highlight a significant role of glutamate/GABA cotransmission in regulating the input-output dynamics of DG circuits.
Keywords: GABA; cotransmission; glutamate; hypothalamus; long-term potentiation; supramammillary nucleus.
Copyright © 2021 Ajibola et al.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
