Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models
- PMID: 34380768
- PMCID: PMC7612153
- DOI: 10.1126/scitranslmed.abd1616
Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models
Abstract
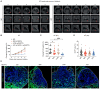
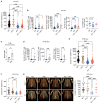
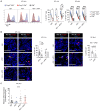
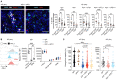
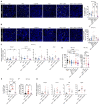
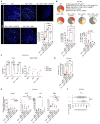
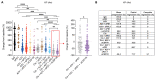
Immune checkpoint blockade (ICB) with PD-1 or PD-L1 antibodies has been approved for the treatment of non-small cell lung cancer (NSCLC). However, only a minority of patients respond, and sustained remissions are rare. Both chemotherapy and antiangiogenic drugs may improve the efficacy of ICB in mouse tumor models and patients with cancer. Here, we used genetically engineered mouse models of Kras G12D/+;p53 -/- NSCLC, including a mismatch repair-deficient variant (Kras G12D/+;p53 -/-;Msh2 -/-) with higher mutational burden, and longitudinal imaging to study tumor response and resistance to combinations of ICB, antiangiogenic therapy, and chemotherapy. Antiangiogenic blockade of vascular endothelial growth factor A and angiopoietin-2 markedly slowed progression of autochthonous lung tumors, but contrary to findings in other cancer types, addition of a PD-1 or PD-L1 antibody was not beneficial and even accelerated progression of a fraction of the tumors. We found that antiangiogenic treatment facilitated tumor infiltration by PD-1+ regulatory T cells (Tregs), which were more efficiently targeted by the PD-1 antibody than CD8+ T cells. Both tumor-associated macrophages (TAMs) of monocyte origin, which are colony-stimulating factor 1 receptor (CSF1R) dependent, and TAMs of alveolar origin, which are sensitive to cisplatin, contributed to establish a transforming growth factor-β-rich tumor microenvironment that supported PD-1+ Tregs Dual TAM targeting with a combination of a CSF1R inhibitor and cisplatin abated Tregs, redirected the PD-1 antibody to CD8+ T cells, and improved the efficacy of antiangiogenic immunotherapy, achieving regression of most tumors.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
-
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. - PubMed
-
- Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, Socinski MA. Third-Generation Chemotherapy Agents in the Treatment of Advanced Non-small Cell Lung Cancer: A Meta-Analysis. J Thorac Oncol. 2007;2:845–853. - PubMed
-
- Soria J-C, Wu Y-L, Nakagawa K, Kim S-W, Yang J-J, Ahn M-J, Wang J, Yang JC-H, Lu Y, Atagi S, Ponce S, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. - PubMed
-
- Soria J-C, Marabelle A, Brahmer JR, Gettinger S. Immune Checkpoint Modulation for Non–Small Cell Lung Cancer. Clin Cancer Res. 2015;21:2256–2262. - PubMed
-
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

