Downregulation of miR-491-5p promotes neovascularization after traumatic brain injury
- PMID: 34380897
- PMCID: PMC8504397
- DOI: 10.4103/1673-5374.314326
Downregulation of miR-491-5p promotes neovascularization after traumatic brain injury
Abstract
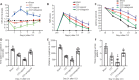
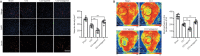
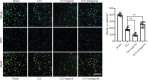
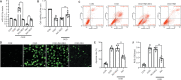
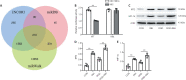
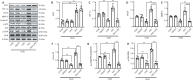
MicroRNA-491-5p (miR-491-5p) plays an important role in regulating cell proliferation and migration; however, the effect of miR-491-5p on neovascularization after traumatic brain injury remains poorly understood. In this study, a controlled cortical injury model in C57BL/6 mice and an oxygen-glucose deprivation model in microvascular endothelial cells derived from mouse brain were established to simulate traumatic brain injury in vivo and in vitro, respectively. In the in vivo model, quantitative real-time-polymerase chain reaction results showed that the expression of miR-491-5p increased or decreased following the intracerebroventricular injection of an miR-491-5p agomir or antagomir, respectively, and the expression of miR-491-5p decreased slightly after traumatic brain injury. To detect the neuroprotective effects of miR-491-p, neurological severity scores, Morris water maze test, laser speckle techniques, and immunofluorescence staining were assessed, and the results revealed that miR-491-5p downregulation alleviated neurological dysfunction, promoted the recovery of regional cerebral blood flow, increased the number of lectin-stained microvessels, and increased the survival of neurons after traumatic brain injury. During the in vitro experiments, the potential mechanism of miR-491-5p on neovascularization was explored through quantitative real-time-polymerase chain reaction, which showed that miR-491-5p expression increased or decreased in brain microvascular endothelial cells after transfection with an miR-491-5p mimic or inhibitor, respectively. Dual-luciferase reporter and western blot assays verified that metallothionein-2 was a target gene for miR-491-5p. Cell counting kit 8 (CCK-8) assay, flow cytometry, and 2?,7?-dichlorofluorescein diacetate (DCFH-DA) assay results confirmed that the downregulation of miR-491-5p increased brain microvascular endothelial cell viability, reduced cell apoptosis, and alleviated oxidative stress under oxygen-glucose deprivation conditions. Cell scratch assay, Transwell assay, tube formation assay, and western blot assay results demonstrated that miR-491-5p downregulation promoted the migration, proliferation, and tube formation of brain microvascular endothelial cells through a metallothionein-2-dependent hypoxia-inducible factor-1α/vascular endothelial growth factor pathway. These findings confirmed that miR-491-5p downregulation promotes neovascularization, restores cerebral blood flow, and improves the recovery of neurological function after traumatic brain injury. The mechanism may be mediated through a metallothionein-2-dependent hypoxia-inducible factor-1α/vascular endothelial growth factor signaling pathway and the alleviation of oxidative stress. All procedures were approved by Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, China (approval No. 2020-304) on June 22, 2020.
Keywords: brain injury; cell migration; cell proliferation; endothelial cell; hypoxia-inducible factor-1 alpha; metallothionein 2; microRNA; neovascularization; neurons; vascular endothelial growth factor.
Conflict of interest statement
None
Figures

References
-
- Bai X, Geng J, Li X, Wan J, Liu J, Zhou Z, Liu X. Long noncoding RNA LINC01619 regulates MicroRNA-27a/Forkhead Box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxid Redox Signal. 2018;29:355–376. - PubMed
-
- Chin HK, Horng CT, Liu YS, Lu CC, Su CY, Chen PS, Chiu HY, Tsai FJ, Shieh PC, Yang JS. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol Rep. 2018;39:2351–2357. - PubMed
LinkOut - more resources
Full Text Sources

