Low-dose rapamycin does not impair vascular integrity and tubular regeneration after kidney transplantation in rats
- PMID: 34381142
- PMCID: PMC8358014
- DOI: 10.1038/s41598-021-95790-1
Low-dose rapamycin does not impair vascular integrity and tubular regeneration after kidney transplantation in rats
Abstract
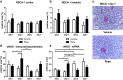
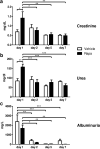
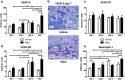
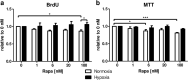
mTOR inhibitors offer advantages after kidney transplantation including antiviral and antitumor activity besides facilitating low calcineurin inhibitor exposure to reduce nephrotoxicity. Concerns about adverse effects due to antiproliferative and antiangiogenic properties have limited their clinical use particularly early after transplantation. Interference with vascular endothelial growth factor (VEGF)-A, important for physiologic functioning of renal endothelial cells and tubular epithelium, has been implicated in detrimental renal effects of mTOR inhibitors. Low doses of Rapamycin (loading dose 3 mg/kg bodyweight, daily doses 1.5 mg/kg bodyweight) were administered in an allogenic rat kidney transplantation model resulting in a mean through concentration of 4.30 ng/mL. Glomerular and peritubular capillaries, tubular cell proliferation, or functional recovery from preservation/reperfusion injury were not compromised in comparison to vehicle treated animals. VEGF-A, VEGF receptor 2, and the co-receptor Neuropilin-1 were upregulated by Rapamycin within 7 days. Rat proximal tubular cells (RPTC) responded in vitro to hypoxia with increased VEGF-A and VEGF-R1 expression that was not suppressed by Rapamycin at therapeutic concentrations. Rapamycin did not impair proliferation of RPTC under hypoxic conditions. Low-dose Rapamycin early posttransplant does not negatively influence the VEGF network crucial for recovery from preservation/reperfusion injury. Enhancement of VEGF signaling peritransplant holds potential to further improve outcomes.
© 2021. The Author(s).
Conflict of interest statement
Klemens Budde has received research funds and/or honoraria from Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL Behring, Fresenius, Hexal, Hookipa Biotech, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, Roche, Shire, Siemens, Takeda, Veloxis and Vitaeris. All other authors declare no competing interests.
Figures


References
-
- Mallat SG, et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor-based regimen versus a CNI-based regimen: a systematic review and meta-analysis of randomized, controlled trials. Clin. J. Am. Soc. Nephrol. 2017;12:1321–1336. doi: 10.2215/CJN.13221216. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

