Aged skeletal stem cells generate an inflammatory degenerative niche
- PMID: 34381212
- PMCID: PMC8721524
- DOI: 10.1038/s41586-021-03795-7
Aged skeletal stem cells generate an inflammatory degenerative niche
Abstract
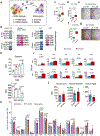
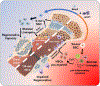
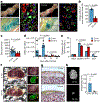
Loss of skeletal integrity during ageing and disease is associated with an imbalance in the opposing actions of osteoblasts and osteoclasts1. Here we show that intrinsic ageing of skeletal stem cells (SSCs)2 in mice alters signalling in the bone marrow niche and skews the differentiation of bone and blood lineages, leading to fragile bones that regenerate poorly. Functionally, aged SSCs have a decreased bone- and cartilage-forming potential but produce more stromal lineages that express high levels of pro-inflammatory and pro-resorptive cytokines. Single-cell RNA-sequencing studies link the functional loss to a diminished transcriptomic diversity of SSCs in aged mice, which thereby contributes to the transformation of the bone marrow niche. Exposure to a youthful circulation through heterochronic parabiosis or systemic reconstitution with young haematopoietic stem cells did not reverse the diminished osteochondrogenic activity of aged SSCs, or improve bone mass or skeletal healing parameters in aged mice. Conversely, the aged SSC lineage promoted osteoclastic activity and myeloid skewing by haematopoietic stem and progenitor cells, suggesting that the ageing of SSCs is a driver of haematopoietic ageing. Deficient bone regeneration in aged mice could only be returned to youthful levels by applying a combinatorial treatment of BMP2 and a CSF1 antagonist locally to fractures, which reactivated aged SSCs and simultaneously ablated the inflammatory, pro-osteoclastic milieu. Our findings provide mechanistic insights into the complex, multifactorial mechanisms that underlie skeletal ageing and offer prospects for rejuvenating the aged skeletal system.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures












Comment in
-
A stem-cell basis for skeletal ageing.Nature. 2021 Sep;597(7875):182-183. doi: 10.1038/d41586-021-02118-0. Nature. 2021. PMID: 34381222 No abstract available.
-
Ageing stem cells hold the key to age-related bone degeneration.Nat Rev Rheumatol. 2021 Oct;17(10):580. doi: 10.1038/s41584-021-00688-x. Nat Rev Rheumatol. 2021. PMID: 34471265 No abstract available.
References
-
- Kenyon CJ The genetics of ageing. Nature 464, 504–512 (2010). - PubMed
-
- Rossi DJ, Jamieson CHM & Weissman IL Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008). - PubMed
-
- Schmich J et al. Induction of reverse development in two marine hydrozoans. Int. J. Dev. Biol 51, 45–56 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 1S10 OD02349701/NH/NIH HHS/United States
- R21 DE019274/DE/NIDCR NIH HHS/United States
- K99 AG049958/AG/NIA NIH HHS/United States
- R01 DE026730/DE/NIDCR NIH HHS/United States
- 1K99 AG066963/NH/NIH HHS/United States
- R00 AG049958/AG/NIA NIH HHS/United States
- R01 DE027323/DE/NIDCR NIH HHS/United States
- R00 AG066963/AG/NIA NIH HHS/United States
- R01 GM138385/GM/NIGMS NIH HHS/United States
- U24 DE026914/DE/NIDCR NIH HHS/United States
- R01 DK115600/DK/NIDDK NIH HHS/United States
- R56 DE025597/DE/NIDCR NIH HHS/United States
- R01 DE021683/DE/NIDCR NIH HHS/United States
- UG3 TR003355/TR/NCATS NIH HHS/United States
- U01 HL099776/HL/NHLBI NIH HHS/United States
- R00 CA151673/CA/NCI NIH HHS/United States
- R01 AI155696/AI/NIAID NIH HHS/United States
- K99 AG066963/AG/NIA NIH HHS/United States
- R21 DE024230/DE/NIDCR NIH HHS/United States
- UG3 TR002968/TR/NCATS NIH HHS/United States
- T32 GM119995/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

