Oxytocin neurons enable social transmission of maternal behaviour
- PMID: 34381215
- PMCID: PMC8387235
- DOI: 10.1038/s41586-021-03814-7
Oxytocin neurons enable social transmission of maternal behaviour
Abstract
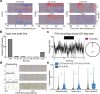
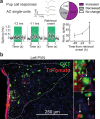
Maternal care, including by non-biological parents, is important for offspring survival1-8. Oxytocin1,2,9-15, which is released by the hypothalamic paraventricular nucleus (PVN), is a critical maternal hormone. In mice, oxytocin enables neuroplasticity in the auditory cortex for maternal recognition of pup distress15. However, it is unclear how initial parental experience promotes hypothalamic signalling and cortical plasticity for reliable maternal care. Here we continuously monitored the behaviour of female virgin mice co-housed with an experienced mother and litter. This documentary approach was synchronized with neural recordings from the virgin PVN, including oxytocin neurons. These cells were activated as virgins were enlisted in maternal care by experienced mothers, who shepherded virgins into the nest and demonstrated pup retrieval. Virgins visually observed maternal retrieval, which activated PVN oxytocin neurons and promoted alloparenting. Thus rodents can acquire maternal behaviour by social transmission, providing a mechanism for adapting the brains of adult caregivers to infant needs via endogenous oxytocin.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Wilson, E. O. Sociobiology: The New Synthesis (Harvard Univ. Press, 1975).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

