DNA interference states of the hypercompact CRISPR-CasΦ effector
- PMID: 34381246
- PMCID: PMC8496406
- DOI: 10.1038/s41594-021-00632-3
DNA interference states of the hypercompact CRISPR-CasΦ effector
Abstract
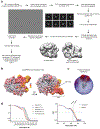
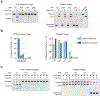
CRISPR-CasΦ, a small RNA-guided enzyme found uniquely in bacteriophages, achieves programmable DNA cutting as well as genome editing. To investigate how the hypercompact enzyme recognizes and cleaves double-stranded DNA, we determined cryo-EM structures of CasΦ (Cas12j) in pre- and post-DNA-binding states. The structures reveal a streamlined protein architecture that tightly encircles the CRISPR RNA and DNA target to capture, unwind and cleave DNA. Comparison of the pre- and post-DNA-binding states reveals how the protein rearranges for DNA cleavage upon target recognition. On the basis of these structures, we created and tested mutant forms of CasΦ that cut DNA up to 20-fold faster relative to wild type, showing how this system may be naturally attenuated to improve the fidelity of DNA interference. The structural and mechanistic insights into how CasΦ binds and cleaves DNA should allow for protein engineering for both in vitro diagnostics and genome editing.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures










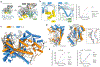

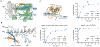

References
METHODS REFERENCES
-
- Punjani A, Zhang H & Fleet DJ Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020). - PubMed
-
- Emsley P & Cowtan K Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60, 2126–2132 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

