An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies
- PMID: 34381282
- PMCID: PMC8343372
- DOI: 10.1016/j.microc.2021.106718
An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies
Abstract
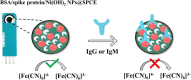
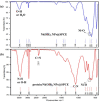
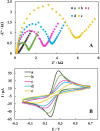
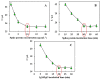
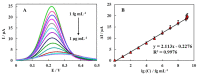
As a promising approach for serological tests, the present study aimed at designing a robust electrochemical biosensor for selective and quantitative analysis of SARS-CoV-2-specific viral antibodies. In our proposed strategy, recombinant SARS-CoV-2 spike protein antigen (spike protein) was used as a specific receptor to detect SARS-CoV-2-specific viral antibodies. In this sense, with a layer of nickel hydroxide nanoparticles (Ni(OH)2 NPs), the screen-printed carbon electrode (SPCE) surface was directly electrodeposited to ensure better loading of spike protein on the surface of SPCE. The differential pulse voltammetry (DPV) showed signals which were inversely proportional to the concentrations of the antibody (from 1 fg mL-1 L to 1 µg mL-1) via a specific and stable binding reaction. The assay was performed in 20 min with a low detection limit of 0.3 fg mL-1. This biodevice had high sensitivity and specificity as compared to non-specific antibodies. Moreover, it can be regarded as a highly sensitive immunological diagnostic method for SARS-CoV-2 antibody in which no labeling is required. The fabricated hand-held biodevice showed an average satisfactory recovery rate of ~99-103% for the determination of antibodies in real blood serum samples with the possibility of being widely used in individual serological qualitative monitoring. Also, the biodevice was tested using real patients and healthy people samples, where the results are already confirmed using the enzyme-linked immunosorbent assay (ELISA) procedure, and showed satisfactory results.
Keywords: Biodevice; Nickel hydroxide nanoparticles; SARS‐CoV‐2; SARS‐CoV‐2-specific viral antibody; Screen-printed carbon electrode; Spike protein.
© 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Determination of rSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures.Biosensors (Basel). 2022 Aug 3;12(8):593. doi: 10.3390/bios12080593. Biosensors (Basel). 2022. PMID: 36004989 Free PMC article.
-
Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein.Mikrochim Acta. 2021 Mar 2;188(3):105. doi: 10.1007/s00604-021-04762-9. Mikrochim Acta. 2021. PMID: 33651173 Free PMC article.
-
Hydroxyapatite-Gold Modified Screen-Printed Carbon Electrode for Selective SARS-CoV-2 Antibody Immunosensor.ACS Appl Bio Mater. 2024 Feb 19;7(2):950-960. doi: 10.1021/acsabm.3c00953. Epub 2024 Feb 2. ACS Appl Bio Mater. 2024. PMID: 38303668
-
Architecting of a biodevice based on a screen-printed carbon electrode modified with the NiONP nanolayer and aptamer in BCM-7 detection.Colloids Surf B Biointerfaces. 2020 Jun;190:110932. doi: 10.1016/j.colsurfb.2020.110932. Epub 2020 Mar 3. Colloids Surf B Biointerfaces. 2020. PMID: 32163843
-
Electrochemical biosensing based comparative study of monoclonal antibodies against SARS-CoV-2 nucleocapsid protein.Sci Total Environ. 2024 Jan 15;908:168154. doi: 10.1016/j.scitotenv.2023.168154. Epub 2023 Nov 1. Sci Total Environ. 2024. PMID: 37923263
Cited by
-
A review post-vaccination SARS-CoV-2 serological test: Method and antibody titer response.Anal Biochem. 2022 Dec 1;658:114902. doi: 10.1016/j.ab.2022.114902. Epub 2022 Sep 17. Anal Biochem. 2022. PMID: 36122603 Free PMC article. Review.
-
Advances in paper-based electrochemical immunosensors: review of fabrication strategies and biomedical applications.R Soc Open Sci. 2023 Nov 29;10(11):230940. doi: 10.1098/rsos.230940. eCollection 2023 Nov. R Soc Open Sci. 2023. PMID: 38034121 Free PMC article. Review.
-
Sensitive sandwich-type electrochemical SARS-CoV‑2 nucleocapsid protein immunosensor.Mikrochim Acta. 2021 Nov 23;188(12):425. doi: 10.1007/s00604-021-05092-6. Mikrochim Acta. 2021. PMID: 34812927 Free PMC article.
-
Advances in Biosensing Technologies for Diagnosis of COVID-19.Biosensors (Basel). 2022 Oct 20;12(10):898. doi: 10.3390/bios12100898. Biosensors (Basel). 2022. PMID: 36291035 Free PMC article. Review.
-
Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody.Diagnostics (Basel). 2022 Oct 27;12(11):2612. doi: 10.3390/diagnostics12112612. Diagnostics (Basel). 2022. PMID: 36359457 Free PMC article.
References
-
- C.P.E.R.E. Novel, The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China, Zhonghua Liu Xing Bing Xue Za Zhi= Zhonghua Liuxingbingxue Zazhi. 41 (2020) 145. - PubMed
-
- Fani M., Teimoori A., Ghafari S. Comparison of the COVID-2019 (SARS-CoV-2) pathogenesis with SARS-CoV and MERS-CoV infections. Future Virol. 2020;15(5):317–323.
-
- W.H.O. Coronavirus, https://covid19. who. int, (2020).
-
- G. Vogel, New blood tests for antibodies could show true scale of coronavirus pandemic, Sci. Mag. AAAS, DC, USA. (2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
