Laboratory capacity assessments in 25 African countries at high risk of yellow fever, August-December 2018
- PMID: 34381546
- PMCID: PMC8325472
- DOI: 10.11604/pamj.2021.38.402.28886
Laboratory capacity assessments in 25 African countries at high risk of yellow fever, August-December 2018
Abstract
Introduction: accurate and timely laboratory diagnosis of yellow fever (YF) is critical to the Eliminate Yellow Fever Epidemics (EYE) strategy. Gavi, the Vaccine Alliance recognized the need to support and build capacity in the national and regional laboratories in the Global YF Laboratory Network (GYFLN) as part of this strategy.
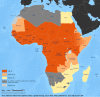
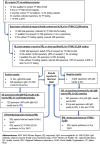
Methods: to better understand current capacity, gaps and needs of the GYFLN laboratories in Africa, assessments were carried out in national and regional reference laboratories in the 25 African countries at high risk for YF outbreaks that were eligible for new financial support from Gavi.
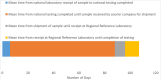
Results: the assessments found that the GYFLN in Africa has high capacity but 21% of specimens were not tested due to lack of testing kits or reagents and approximately 50% of presumptive YF cases were not confirmed at the regional reference laboratory due to problems with shipping.
Conclusion: the laboratory assessments helped to document the baseline capacities of these laboratories prior to Gavi funding to support strengthening YF laboratories.
Keywords: Yellow fever; diagnostics; laboratory; testing.
Copyright: Barbara Wilmot Johnson et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Burke D, Monath TP. Lippincott William and Wilkins. 2001. Flaviviruses Fields Virology Philadelphia; pp. 1043–1125.
-
- Burke D, Leake C. 1988. Yellow fever. In: The Arboviruses: Epidemiology and Ecology. Boca Raton, FL. CDC Press: 63-92. Accessed on March 15th 2021.
-
- World Health Organization Vaccines and vaccination against yellow fever: WHO position paper, June 2013-recommendations. Vaccine. 2015;33(1):76–77. - PubMed
-
- Ahmed QA, Memish ZA. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47(2):92–96. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
