Reduced Susceptibility to Metronidazole Is Associated With Initial Clinical Failure in Clostridioides difficile Infection
- PMID: 34381844
- PMCID: PMC8351808
- DOI: 10.1093/ofid/ofab365
Reduced Susceptibility to Metronidazole Is Associated With Initial Clinical Failure in Clostridioides difficile Infection
Abstract
Background: Clinical studies have demonstrated inferior cure rates when metronidazole (MTZ) is used to treat Clostridioides difficile infection (CDI). We hypothesized that a newly identified, heme-inducible form of reduced MTZ susceptibility in C. difficile leads to higher odds of initial clinical failure in patients with CDI treated with MTZ.
Methods: This multicenter cohort study included adults diagnosed with CDI between 2017 and 2018. C. difficile isolated from stool samples underwent agar dilution MTZ susceptibility testing with incorporation of fresh heme. Blinded investigators reviewed medical records for initial clinical failure and other relevant clinical variables. Classification and regression tree (CART) analysis was used to identify the MTZ minimum inhibitory concentration (MIC) breakpoint that was predictive of initial clinical failure. Results were confirmed using univariate and multivariable logistic regression analyses to account for potential confounders.
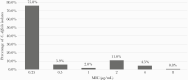
Results: Of the 356 patients included, 72% received MTZ-based therapy and 27% experienced initial clinical failure. CART analysis identified an MTZ MIC ≥1 µg/mL above which patients had a higher rate of initial clinical failure. MTZ MICs ranged from 0.25 to 8 µg/mL (MIC50/90 = 0.25/2 µg/mL), and approximately 18% of isolates had MTZ MICs ≥1 µg/mL. In multivariable analysis, an MTZ MIC ≥1 µg/mL was an independent predictor of initial clinical failure in patients receiving an MTZ-based treatment regimen (odds ratio, 2.27 [95% confidence interval, 1.18-4.34]).
Conclusions: Using a reproducible method to determine C. difficile MICs to MTZ, a breakpoint of ≥1 µg/mL identified patients at higher risk of initial clinical failure.
Keywords: Clostridium difficile; antibiotic resistance; antimicrobial resistance; heme; susceptibility testing.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Hall AJ, Curns AT, McDonald LC, et al. . The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55:216–23. - PubMed
-
- Cohen SH, Gerding DN, Johnson S, et al. . Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. - PubMed
-
- Surawicz CM, Brandt LJ, Binion DG, et al. . Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 99. - PubMed

