Transgenic modeling of Ndr2 gene amplification reveals disturbance of hippocampus circuitry and function
- PMID: 34381982
- PMCID: PMC8340122
- DOI: 10.1016/j.isci.2021.102868
Transgenic modeling of Ndr2 gene amplification reveals disturbance of hippocampus circuitry and function
Abstract
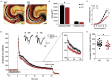
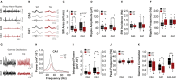
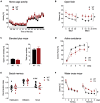
Duplications and deletions of short chromosomal fragments are increasingly recognized as the cause for rare neurodevelopmental conditions and disorders. The NDR2 gene encodes a protein kinase important for neuronal development and is part of a microduplication region on chromosome 12 that is associated with intellectual disabilities, autism, and epilepsy. We developed a conditional transgenic mouse with increased Ndr2 expression in postmigratory forebrain neurons to study the consequences of an increased gene dosage of this Hippo pathway kinase on brain circuitry and cognitive functions. Our analysis reveals reduced terminal fields and synaptic transmission of hippocampal mossy fibers, altered hippocampal network activity, and deficits in mossy fiber-dependent behaviors. Reduced doublecortin expression and protein interactome analysis indicate that transgenic Ndr2 disturbs the maturation of granule cells in the dentate gyrus. Together, our data suggest that increased expression of Ndr2 may critically contribute to the development of intellectual disabilities upon gene amplification.
Keywords: genetics; neuroscience; sensory neuroscience.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Silencing of dentate gyrus inhibits mossy fiber sprouting and prevents epileptogenesis through NDR2 kinase in pentylenetetrazole kindling rat model of TLE.PLoS One. 2023 Apr 12;18(4):e0284359. doi: 10.1371/journal.pone.0284359. eCollection 2023. PLoS One. 2023. PMID: 37043471 Free PMC article.
-
Regulation of hippocampal mossy fiber-CA3 synapse function by a Bcl11b/C1ql2/Nrxn3(25b+) pathway.Elife. 2024 Feb 15;12:RP89854. doi: 10.7554/eLife.89854. Elife. 2024. PMID: 38358390 Free PMC article.
-
Altered morphology of hippocampal dentate granule cell presynaptic and postsynaptic terminals following conditional deletion of TrkB.Hippocampus. 2008;18(7):668-78. doi: 10.1002/hipo.20426. Hippocampus. 2008. PMID: 18398849 Free PMC article.
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
-
Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders.Mol Neurobiol. 2009 Feb;39(1):24-36. doi: 10.1007/s12035-008-8049-5. Epub 2009 Jan 8. Mol Neurobiol. 2009. PMID: 19130314 Review.
Cited by
-
The Serine/Threonine Kinase NDR2 Regulates Integrin Signaling, Synapse Formation, and Synaptic Plasticity in the Hippocampus.J Neurochem. 2025 Jun;169(6):e70094. doi: 10.1111/jnc.70094. J Neurochem. 2025. PMID: 40439020 Free PMC article.
-
The NDR family of kinases: essential regulators of aging.Front Mol Neurosci. 2024 May 13;17:1371086. doi: 10.3389/fnmol.2024.1371086. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38803357 Free PMC article. Review.
-
Silencing of dentate gyrus inhibits mossy fiber sprouting and prevents epileptogenesis through NDR2 kinase in pentylenetetrazole kindling rat model of TLE.PLoS One. 2023 Apr 12;18(4):e0284359. doi: 10.1371/journal.pone.0284359. eCollection 2023. PLoS One. 2023. PMID: 37043471 Free PMC article.
References
-
- Albrecht A., Caliskan G., Oitzl M.S., Heinemann U., Stork O. Long-lasting increase of corticosterone after fear memory reactivation: anxiolytic effects and network activity modulation in the ventral hippocampus. Neuropsychopharmacology. 2013;38:386–394. doi: 10.1038/npp.2012.192. - DOI - PMC - PubMed
-
- Andres-Alonso M., Ammar M.R., Butnaru I., Gomes G.M., Acuña Sanhueza G., Raman R., Yuanxiang P., Borgmeyer M., Lopez-Rojas J., Raza S.A. SIPA1L2 controls trafficking and local signaling of TrkB-containing amphisomes at presynaptic terminals. Nat. Commun. 2019;10:5448. doi: 10.1038/s41467-019-13224-z. - DOI - PMC - PubMed
-
- Arruda-Carvalho M., Restivo L., Guskjolen A., Epp J.R., Elgersma Y., Josselyn S.A., Frankland P.W. Conditional deletion of -CaMKII impairs integration of adult-generated granule cells into dentate gyrus circuits and hippocampus-dependent learning. J. Neurosci. 2014;34:11919–11928. doi: 10.1523/JNEUROSCI.0652-14.2014. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

