Unusual Temperature Dependence of Bandgap in 2D Inorganic Lead-Halide Perovskite Nanoplatelets
- PMID: 34382362
- PMCID: PMC8498867
- DOI: 10.1002/advs.202100084
Unusual Temperature Dependence of Bandgap in 2D Inorganic Lead-Halide Perovskite Nanoplatelets
Abstract
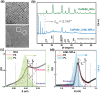
Understanding the origin of temperature-dependent bandgap in inorganic lead-halide perovskites is essential and important for their applications in photovoltaics and optoelectronics. Herein, it is found that the temperature dependence of bandgap in CsPbBr3 perovskites is variable with material dimensionality. In contrast to the monotonous redshift ordinarily observed in bulk-like CsPbBr3 nanocrystals (NCs), the bandgap of 2D CsPbBr3 nanoplatelets (NPLs) exhibits an initial blueshift then redshift trend with decreasing temperature (290-10 K). The Bose-Einstein two-oscillator modeling manifests that the blueshift-redshift crossover of bandgap in the NPLs is attributed to the significantly larger weight of contribution from electron-optical phonon interaction to the bandgap renormalization in the NPLs than in the NCs. These new findings may gain deep insights into the origin of bandgap shift with temperature for both fundamentals and applications of perovskite semiconductor materials.
Keywords: blueshift-redshift crossover; inorganic lead-halide perovskites; material dimensionality; temperature-dependent bandgap.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites.Sci Adv. 2016 Oct 28;2(10):e1601156. doi: 10.1126/sciadv.1601156. eCollection 2016 Oct. Sci Adv. 2016. PMID: 27819049 Free PMC article.
-
Absence of Anomalous Electron-Phonon Coupling in the Near-Ambient Gap Temperature Renormalization of CsPbBr3 Nanocrystals.J Phys Chem C Nanomater Interfaces. 2024 Dec 19;129(1):453-463. doi: 10.1021/acs.jpcc.4c06265. eCollection 2025 Jan 9. J Phys Chem C Nanomater Interfaces. 2024. PMID: 40115613 Free PMC article.
-
Dilution-Induced Formation of Hybrid Perovskite Nanoplatelets.ACS Nano. 2016 Dec 27;10(12):10936-10944. doi: 10.1021/acsnano.6b05649. Epub 2016 Dec 2. ACS Nano. 2016. PMID: 28024369
-
Colloidal Metal-Halide Perovskite Nanoplatelets: Thickness-Controlled Synthesis, Properties, and Application in Light-Emitting Diodes.Adv Mater. 2022 Mar;34(10):e2107105. doi: 10.1002/adma.202107105. Epub 2022 Jan 28. Adv Mater. 2022. PMID: 34775643 Review.
-
Two-dimensional metal halide perovskites and their heterostructures: from synthesis to applications.Nanophotonics. 2023 Mar 22;12(9):1643-1710. doi: 10.1515/nanoph-2022-0797. eCollection 2023 Apr. Nanophotonics. 2023. PMID: 39634119 Free PMC article. Review.
Cited by
-
Advances in Emerging Photonic Memristive and Memristive-Like Devices.Adv Sci (Weinh). 2022 Oct;9(28):e2105577. doi: 10.1002/advs.202105577. Epub 2022 Aug 9. Adv Sci (Weinh). 2022. PMID: 35945187 Free PMC article. Review.
-
A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional-Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition.Nanomaterials (Basel). 2023 Oct 7;13(19):2723. doi: 10.3390/nano13192723. Nanomaterials (Basel). 2023. PMID: 37836364 Free PMC article.
-
Revealing Two Distinct Formation Pathways of 2D Wurtzite-CdSe Nanocrystals Using In Situ X-Ray Scattering.Adv Sci (Weinh). 2024 Feb;11(6):e2307600. doi: 10.1002/advs.202307600. Epub 2023 Dec 10. Adv Sci (Weinh). 2024. PMID: 38072639 Free PMC article.
References
-
- a) Zhang J. R., Hodes G., Jin Z. W., Liu S. F., Angew. Chem., Int. Ed. 2019, 58, 15596; - PubMed
- b) Zhu M. H., Duan Y. Q., Liu N., Li H. G., Li J. H., Du P. P., Tan Z. F., Niu G. D., Gao L., Huang Y. A., Yin Z. P., Tang J., Adv. Funct. Mater. 2019, 29, 1903294;
- c) Chen Q. S., Wu J., Ou X. Y., Huang B., Almutlaq J., Zhumekenov A. A., Guan X. W., Han S. Y., Liang L. L., Yi Z. G., Li J., Xie X. J., Wang Y., Li Y., Fan D. Y., Teh D. B. L., All A. H., Mohammed O. F., Bakr O. M., Wu T., Bettinelli M., Yang H., Huang W., Liu X. G., Nature 2018, 561, 88; - PubMed
- d) Lin K. B., Xing J., Quan L. N., de Arquer F. P. G., Gong X. W., Lu J. X., Xie L. Q., Zhao W. J., Zhang D., Yan C. Z., Li W., Liu X. Y., Lu Y., Kirman J., Sargent E. H., Xiong Q. H., Wei Z. H., Nature 2018, 562, 245; - PubMed
- e) Pan G. C., Bai X., Yang D. W., Chen X., Jing P. T., Qu S. N., Zhang L. J., Zhou D. L., Zhu J. Y., Xu W., Dong B., Song H. W., Nano Lett. 2017, 17, 8005; - PubMed
- f) Kroupa D. M., Roh J. Y., Milstein T. J., Creutz S. E., Gamelin D. R., ACS Energy Lett. 2018, 3, 2390;
- g) Yong Z. J., Guo S. Q., Ma J. P., Zhang J. Y., Li Z. Y., Chen Y. M., Zhang B. B., Zhou Y., Shu J., Gu J. L., Zheng L. R., Bakr O. M., Sun H. T., J. Am. Chem. Soc. 2018, 140, 9942; - PubMed
- h) Yao E. P., Yang Z. L., Meng L., Sun P. Y., Dong S. Q., Yang Y., Yang Y., Adv. Mater. 2017, 29, 1606859;
- i) Yao J.‐S., Ge J., Wang K.‐H., Zhang G., Zhu B. S., Chen C., Zhang Q., Luo Y., Yu S.‐H., Yao H.‐B., J. Am. Chem. Soc. 2019, 141, 2069; - PubMed
- j) Cheng P., Sun L., Feng L., Yang S., Yang Y., Zheng D., Zhao Y., Sang Y., Zhang R., Wei D., Deng W., Han K., Angew. Chem., Int. Ed. 2019, 131, 16233. - PubMed
-
- a) Weidman M. C., Goodman A. J., Tisdale W. A., Chem. Mater. 2017, 29, 5019;
- b) Fu Y., Zhu H., Chen J., Hautzinger M. P., Zhu X. Y., Jin S., Nat. Rev. Mater. 2019, 4, 169.
-
- a) Diroll B. T., Zhou H., Schaller R. D., Adv. Funct. Mater. 2018, 28, 1800945;
- b) Lo S. S., Khan Y., Jones M., Scholes G. D., J. Chem. Phys. 2009, 131, 084714; - PubMed
- c) Li Y., Shi Z., Lei L., Zhang F., Ma Z., Wu D., Xu T., Tian Y., Zhang Y., Du G., Shan C., Li X., Chem. Mater. 2018, 30, 6744; - PubMed
- d) Fang Z. S., He H. P., Gan L., Li J., Ye Z. Z., Adv. Sci. 2018, 5, 1800736; - PMC - PubMed
- e) Tamarat P., Bodnarchuk M. I., Trebbia J.‐B., Erni R., Kovalenko M. V., Even J., Lounis B., Nat. Mater. 2019, 18, 717. - PubMed
-
- a) Li X. M., Wu Y., Zhang S. L., Cai B., Gu Y., Song J. Z., Zeng H. B., Adv. Funct. Mater. 2016, 26, 2435;
- b) Quan L. N., Garcia de Arquer F. P., Sabatini R. P., Sargent E. H., Adv. Mater. 2018, 30, 1801996. - PubMed
-
- a) Zhang Q., Yin Y. D., ACS Cent. Sci. 2018, 4, 668; - PMC - PubMed
- b) Hu X. L., Zhou H., Jiang Z. Y., Wang X., Yuan S. P., Lan J. Y., Fu Y. P., Zhang X. H., Zheng W. H., Wang X. X., Zhu X. L., Liao L., Xu G. Z., Jin S., Pan A. L., ACS Nano 2017, 11, 9869; - PubMed
- c) Xu K., Meijerink A., Chem. Mater. 2018, 30, 5346. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
