N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle
- PMID: 34382372
- PMCID: PMC8498914
- DOI: 10.1002/advs.202100584
N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle
Abstract
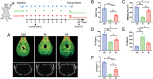
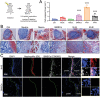
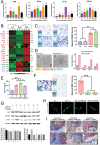
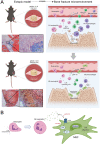
The role of neutrophils in bone regeneration remains elusive. In this study, it is shown that intramuscular implantation of interleukin-8 (IL-8) (commonly recognized as a chemotactic cytokine for neutrophils) at different levels lead to outcomes resembling those of fracture hematoma at various stages. Ectopic endochondral ossification is induced by certain levels of IL-8, during which neutrophils are recruited to the implanted site and are N2-polarized, which then secrete stromal cell-derived factor-1α (SDF-1α) for bone mesenchymal stem cell (BMSC) chemotaxis via the SDF-1/CXCR4 (C-X-C motif chemokine receptor 4) axis and its downstream phosphatidylinositol 3'-kinase (PI3K)/Akt pathway and β-catenin-mediated migration. Neutrophils are pivotal for recruiting and orchestrating innate and adaptive immunocytes, as well as BMSCs at the initial stage of bone healing and regeneration. The results in this study delineate the mechanism of neutrophil-initiated bone regeneration and interaction between neutrophils and BMSCs, and innate and adaptive immunities. This work lays the foundation for research in the fields of bone regenerative therapy and biomaterial development, and might inspire further research into novel therapeutic options.
Keywords: bone regeneration; interleukin-8; neutrophils; stem cell recruitment; stromal cell-derived factor-1α.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wells J. M., Watt F. M., Nature 2018, 557, 322. - PubMed
-
- a) Petite H., Viateau V., Bensaid W., Meunier A., de Pollak C., Bourguignon M., Oudina K., Sedel L., Guillemin G., Nat. Biotechnol. 2000, 18, 959; - PubMed
- b) Harada S., Rodan G. A., Nature 2003, 423, 349; - PubMed
- c) Grayson W. L., Bunnell B. A., Martin E., Frazier T., Hung B. P., Gimble J. M., Nat. Rev. Endocrinol. 2015, 11, 140. - PMC - PubMed
-
- Forbes S. J., Rosenthal N., Nat. Med. 2014, 20, 857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
