Cytomegalovirus seroprevalence among blood donors: a systematic review and meta-analysis
- PMID: 34382466
- PMCID: PMC8366145
- DOI: 10.1177/03000605211034656
Cytomegalovirus seroprevalence among blood donors: a systematic review and meta-analysis
Abstract
Background: Screening for cytomegalovirus (CMV)-specific antibodies is not routine in some settings. Thus, transfusion of blood products poses risks for susceptible individuals.
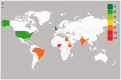
Objectives: To investigate the global pooled CMV seroprevalence among volunteer blood donors.
Methods: This systematic review and meta-analysis was performed according to PRISMA guidelines. The databases searched included Embase, Google Scholar, Medline, PubMed, Web of Science, and Cochrane Library. Data were extracted independently and analyzed using STATA version 11.
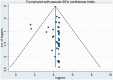
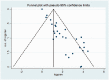
Results: The global seroprevalence of CMV IgG, CMV IgM, and both CMV IgM and IgG was 83.16% (95% confidence interval [CI]: 78.55-87.77%, I2 = 99.5%), 13.77% (95% CI: 11.59-15.95%, I2 = 98.8%), and 23.78% (95% CI: 10.50-37.06%, I2 = 98.7), respectively.
Conclusion: The global seroprevalence of CMV was high among blood donors. Therefore, regular CMV screening should be conducted to identify CMV-seronegative blood donors.
Keywords: Blood donor; cytomegalovirus; immunoglobulin G; immunoglobulin M; seroprevalence; systematic review.
Conflict of interest statement
Figures




References
-
- Nogalski MT, Collins-McMillen D, Yurochko AD. Overview of human cytomegalovirus pathogenesis. Human Cytomegaloviruses. Springer, 2014, pp. 15–28. - PubMed
-
- Jobier A, Ali MA.Frequency rate of cytomegalovirus antibodies among blood donors in Khartoum state. African Journal of Medical Sciences 2018; 3(2).
-
- Ziemann M, Krueger S, Maier AB, et al.. High prevalence of cytomegalovirus DNA in plasma samples of blood donors in connection with seroconversion. Transfusion 2007; 47: 1972–1983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

